
| |||||||||||||||||||||||||||||||||||||||||||

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��ɽ��ʡɽ����2010��2011ѧ���һ5���¿��������� ���ͣ�013
Ұ���ʹ˾������û����������еļ�Ӫ�����ʺϳ���������������İ����ᣬ��ɫ���ᡣ�������������ͻ�䣬����������Ӧ��ijһ���費�ܽ��У�����ʹijЩ�������ʲ��ܺϳɣ��������ڻ�����������������ij��ѧ�����������ߴ���Ұ���ʹ˾��õ�4�ֲ����ڻ�����������������ͻ���塣��֪A��B��C��D��E�Ǻϳ�ɫ������м��壬ͻ�����ס�������ɫ������������У�������A��E��һ������������������±�������������������������
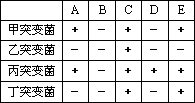
����ʵ�飬�ж�����˵������ȷ���ǣ�
A���������ͨ������ø�ĺϳ������ƴ�л���̣������������������״
B������ͻ���Dz������
C��������Ұ���ʹ˾����ͻ���壬Ҳ��������ͻ������Ұ���ʹ˾�
D���˾���������ϳ�ɫ�����;���ǣ�B��D��A��C��E
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ������ʡ����һ��2011��2012ѧ���һ��ѧ�����п����������� ���ͣ�013
Ұ���ʹ˾����ڻ���������������������������Ұ���ʹ˾��õ�һͻ���꣬��ͻ�����ڻ���������������ʱ�������Ӱ�������������������һʵ�����Ľ��ͣ�����������
A��Ұ���ʹ˾����Ժϳɰ������
B��Ұ���ʹ˾���л���ܲ���Ҫ�������
C����ͻ�������������������ϳ������ø
D����ͻ�����кϳɰ����������ø�Ĺ��ܿ���ɥʧ
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ������ʡ�Ͼ��н�����ѧ2011��������Ĵ�ģ�⿼���������� ���ͣ�071
�������칹ø�ڹ�ҵ��Ӧ�ù㷺��Ϊ��������ȶ��ԣ���ѧ����ȷ����138λ�ʰ���ΪĿ�갱������������칹ø����������ⶨ���ձ䣬�Ը�����138����ʰ���138����ͻ��������������ڴ˾��б�����ͻ�����������칹ø��Ұ���͵����ʷ�Ӧ�¶����10��12�棬ø�����ȶ��Եõ�����ߡ����������ͼʾ�����ش����⣺
��1�����������칹ø���ж����ձ�Ĺ���������_________��_________�ִ����\�����ۺ����á�
��2��1�����ƽ�_________������ɰ�����Ŀ�Ļ�����ֹ�ӡ���ǻ�����ԭ���_________��
��3��Ŀ�Ļ������ⶨ���ձ��Ϊ������䵼��˾��ijɹ��ʣ�����_________�����˾���
��4����ҵ������Ϊ�˳�������������칹ø��������_________������ʹ�ô˼������ŵ��Ǣ�_________����_________��
��5��������������ȣ���������ͻ���Ļ����з��������Լ����_________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2012�����ʡ����һ�и�����ѧ�����п��������ۺ����⣨���ﲿ�֣� ���ͣ��ۺ���
��ÿ��1�֣���14�֣�
����ͻ�������ù�����ڻ����������������ʵ��İ�������������������������ͣ����������������������ͺ�ɫ���ù��ͻ����a��ͻ����b����������ʵ�飺
ʵ��һ������������ͻ����ֱ����������6���������ϣ�����ͻ���겻����l��3��5��
����������������2��4��6�������϶����������������ijɷ����±���ʾ����ͻ����a��
b��Ҫ����A��J�е����������������������
| �������� | �����ɷ� | ���ӵİ��������� |
| �� | | �� |
| �� | | �����¡��á��� |
| �� | ���������� | �¡��š��ơ��� |
| �� | �á��ơ��ȡ��� | |
| �� | �ġ��ǡ��ɡ��� | |
| �� | �����ʶ��� |


 ���� �������� �ϰ��� ����������X
���� �������� �ϰ��� ����������X

 ���� ���� �ϰ��� ������X
���� ���� �ϰ��� ������X
|

| ��� | �� | �� | �� | �� | �� | �� | �� |
| �ɷ� | S | ��NH4��2SO4 | K2HPO4 | MgSO4 | FeSO4 | CaCl2 | H2O |
| ���� | 10g | 0��4g | 4��0g | 9��25g | 0��5g | 0��5g | 100mL |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com