
(9��)��ͼ��ʾϸ���˽ṹģʽͼ�� �˵�����ͨ��
�˵�����ͨ�� �˿���ϸ���˷��ӹ��ܣ�
�˿���ϸ���˷��ӹ��ܣ�
��ͼΪ����צ���ĸϸ���˵���ע��ʵ�飬�����ͼ�ش�����
(1)��Ĥ�� ����֬������ɣ���Ҫ�ɷֻ��� ��ͬ��������Ĥ��ͬ�Ĺ��������� �ԣ���л��ʢ��ϸ������Ĥ�ϵ�  ��Ŀ�࣬���ǵ�������
��Ŀ�࣬���ǵ�������  ������ͼ��֪�˵�����ϸ�������� ����������Ҫ ��(2)ϸ�������Ŵ���Ϣ�⣬��ϸ����л���Ŵ��Ŀ������ģ���Щ������ṹ (��
������ͼ��֪�˵�����ϸ�������� ����������Ҫ ��(2)ϸ�������Ŵ���Ϣ�⣬��ϸ����л���Ŵ��Ŀ������ģ���Щ������ṹ (��
����)�� �أ��ṹ�ڵ������� ��
�أ��ṹ�ڵ������� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��2013-2014ѧ�꽭���һ�и������Ĵ�ģ��������������棩 ���ͣ��ۺ���
(9��)��ͼ���������������ֵ�����ͼ����������������������л���ֿ��Ĺ���������Ա��������������P�����й�(��P+��ʾ����������p-��ʾ���Ĺ�����)�������������\���Դ˻���ı仯�����˼�⣬�������ͼ�ҡ����ͼ�ش������й����⣺

(1)ͼ������ˮ���ش������������ϵ������ ʱ�����յ��ı���IJ������������γɵ��ı���ֲ����ϸ��Ⱦɫ������������ ��
(2)ͼ�������������������������ݵ�ԭ���� ������ͼ����ʾ���������������������ֹ��̺ܷ�������˿��Բ�ȡֲ��ϸ�������� ��������������
(3)����ͼ�ҷ����������������Ϊ���Ĺ�����ĸ���ԭ���� ����֪����øXʶ������ΪCCGG����������øX��ȫ�и�ͼ���п��Ĺ���p-����������ô���Ĺ��Ļ������гɳ���Ϊ �Լ���� �Լ��������Ƭ�ϡ�
(4)����ij���������������������з�������������ĸ���P�����й���������øX��ȫ�и������170��220��290��460�Լ��������Ƭ�Σ���ô���������������ϵĻ������� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2014�����ʡ����8���¿������Ծ��������棩 ���ͣ��ۺ���
(10��)��ͼ��ʾij�ߵȴ��Զ����ڽ���ϸ������ʱ��ͼ�Σ���ͼ��ʾ���������ϸ����Ⱦɫ�弰DNA��Ժ����仯������ͼ�����ݴ����ߺ�ͼ�ش��������⣺


(1)��ͼ�е�A��B�ֱ���ʲô����ʲôʱ�ڣ�A��________________��B��________________��
(2)��ͼ��8���������������̽�_______________��
(3)��ͼϸ���ں���Ⱦɫ�����������________��9��11������ͬԴȾɫ���������________��
(4)����������ϸ����Ⱦɫ����Ϊ20������һ��ϸ����4��5ʱ��Ⱦɫ����ĿΪ________����
(5)ͬԴȾɫ��ķ��뷢��������________��
(6)ʱ��6��11�Ĺ�ͬ����_________________________��
(7)��ͼ��Bϸ����Ӧ��ͼ�е�������________���γɵ���ϸ����________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2011-2012ѧ��ɽ��ʡ�����ڶ���������������Ծ� ���ͣ��ۺ���
(9��)��ͼ��ʾϸ���˽ṹģʽͼ���˵�����ͨ���˿���ϸ���˷��ӹ��ܣ�
��ͼΪ����צ���ĸϸ���˵���ע��ʵ�飬�����ͼ�ش�����
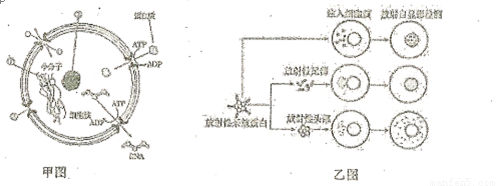
(1)��Ĥ�� ����֬������ɣ���Ҫ�ɷֻ��� ��ͬ��������Ĥ��ͬ�Ĺ��������� �ԣ���л��ʢ��ϸ������Ĥ�ϵ� ��Ŀ�࣬���ǵ������� ������ͼ��֪�˵�����ϸ�������� ����������Ҫ ��(2)ϸ�������Ŵ���Ϣ�⣬��ϸ����л���Ŵ��Ŀ������ģ���Щ������ṹ (��
����)�йأ��ṹ�ڵ������� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��Ķ�����
��ͼ��ʾС��Ҷ��ϸ���ڷ����Ĺ�����ú������������̣�ͼ�Т١��ܱ�ʾ��ع��̣�a��bΪ���������ʣ���ͼ��ʾ�����ֲ�ͬ�¶��£�С�������̼�����������ǿ�ȵĹ�ϵ���������������¶�Ϊ25�棬���ͼ�ش��������⣺
 |

��ͼ���������������������������������� ��ͼ
(1)��ͼ�Т٢����������������ľ��岿λ�ֱ���________________________________��
__________________________________��
(2)��ͼ�۹��̰���____________ ____��_________ _________���ܹ��̲�����b��ٹ��̲�����a�ڹ����ϵ�������b_____________________��a_______________________��
(3)����ͼ֪����B���ΪA��һ��ʱ���С���л���������________________(���ӡ����١�����)����A��ΪB����λ�����λʱ���л��������_________(���ӡ����١�����)��
(4)����ͼ�пɷ��֣�Ӱ��B�������ʵ����ذ���___________��_____________��
(5)�پ��о����֣��������ɺ�ʱ��С���ϸ����Ѹ�ٺϳ�ij�ֻ�ѧ����X�������Ʋ�����ϳɵ�X���䵽ҶƬ���ܵ������Ŀ��ա�������������ʵ�飺�Ӹ�ֲ���ϼ�ȡ��С������״̬һ�µ�3ƬҶ���ֱ�Ҷ���²����ڲ�ͬŨ��X������Һ�У��Է���ҶƬ��X����Ũ���������ų̶�֮��Ĺ�ϵ��һ��ʱ����Բ���й����ݡ�
���Ϸ��������������Ƶĵط�����ָ���������Ը�����
______________________________________________________________________________
______________________________________________________________________________
�ڷ������ƺ���й����ݣ��õ��±��Ľ����(ע��������Խ���������̶�Խ��)
| ���� ����ָ�� | ����Һ��X��Ũ��/mol��m-3 | ||
| 5��10-5 | 5��10-4 | 5��10-3 | |
| ҶƬ��X��Ũ��/mol��g-1(����) | 2.4 | 2.9 | 9.2 |
| ҶƬ�е�������/mol��m-2��a-1 | 0.5 | 0.4 | 0.2 |
�ɴ˿����Ʋ⣬��������Һ��XŨ�ȵ�����ֲ��������ǿ��____________��ԭ����______________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com