
(2011��֣��)����¹�̷��¼�����¶�˲����������̷��������������谷�������谷�׳Ƶ����������ʽΪC3N6H6��������Ϊ66%���ң��ÿ��϶������ⶨ���ϻ�ʳƷ�е����ʺ���ʱ�������������ֳ���Ϊα�����ʡ�����ʳ�ú������谷���̷ۻ�����ֳ����ϵͳ���γɰ��������ʯ���������˵����ȷ����(����)
A�������������ĵ�Ԫ����Ҫ������R����
B�������谷�백�������Ԫ����ͬ��ʳƷ�����������谷������ߺ����ʣ�ʹ����ʳƷͨ���ʼ�����ļ��
C����˫�����Լ�����ţ�̺������谷�����߲���������ɫ������
D�������谷��ϸ������ȥ������������ֽ���������CO2��H2O
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
14��(2011��֣��)�������ƬҶ����0.45 mol/L������Һ�У�ϸ�������ʱڷ��룻����0.35 mol/L������Һ�У�ϸ�����ʹ����ƣ�����0.4 mol/L������Һ�У�ϸ���ƺ�������ʲô�仯�������(����)
A����б�Ƥϸ���Ѿ�����
B��ϸ��Ĥ������ˮ��������ͨ��
C�����Ƿ��ӽ���ϸ��������ƽ��
D��ϸ��ҺŨ��Ϊ0.4 mol/L����
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
35��(2011��֣��)(9��)ͼ1�����ָߵ�����ϸ�������ṹģʽͼ��ͼ2��4��ͼ1�в��ֽṹ�ķŴ�ͼ����ͼ�ش�(�ۡ���������ͼ�б�ţ�________�����ʵ����ݵ�����)
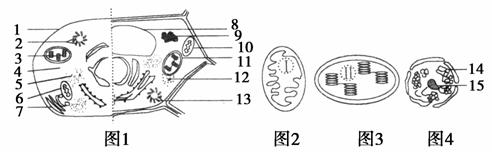
(1)ͼ�еĽṹ1��15�в�Ӧ�ó��ֵ��ǣۡ����ݺͣۡ����ݣ����ܺ���ˮ����ɫ�ص��ǣۡ����ݣ��ṹ��15����ۡ����ݵ��γ��йء�
(2)ͼ2��3��4��ʾ�ڽṹ�ϵ���Ҫ��ͬ����________��ͼ2��3��ʾ�ṹ�����Ʒֱ���________�������н��еĹ��̢�ֱ���________��
(3)��֪����������ֳ��ij����ͬʱ����ͼ2��3��4��ʾ���ֽṹ�������ֽṹ�е�DNA�ܴӸ������ݸ��Ӵ�����______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com