
��8�֣�������Ƥϸ���ϳ���֭�����Ӫ��������ѪҺ��������Ϊţ������Ƥϸ���ϳ��������֭��ʾ��ͼ���±�Ϊţ��֭��ѪҺ�IJ��ֳɷֱȽϡ�
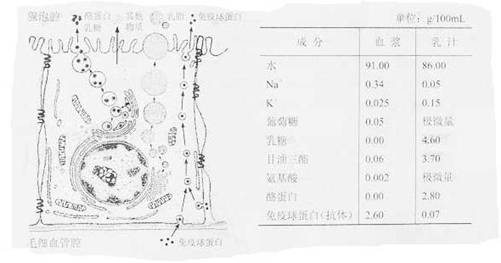
��1����֭��Ƥϸ���������ڻ�����_____________�����е�K+ ��ͨ��_________��ʽת�˵�������Ƥϸ����
��2����Ѫ����ȣ���֭�����еijɷ���___________________��
��3����֬����Ҫ�ɷ��Ǹ����������ϳɸ���������ϸ������______________________��
��4���ϳ���������������Ҫ����Ѫ�����������ñ���Ѫ����______________________ת��������
��5��ͼ���У�������������Ƥϸ���кϳɵ�����������___________________����ţ�鷿������֢ʱ����������Ѫ������֭�еĺ�����__________�������ʵ�������_________________________________��
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��2011����ͨ�ߵ�ѧУ����ȫ��ͳһ�������������� ���ͣ��ۺ���
������Ƥϸ���ϳ���֭�����Ӫ��������ѪҺ��������Ϊţ������Ƥϸ���ϳ��������֭��ʾ��ͼ���±�Ϊţ��֭��ѪҺ�IJ��ֳɷֱȽϡ���λ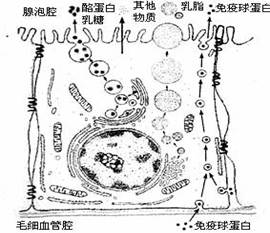
���g/ml
| �ɷ� | Ѫ�� | ��֭ |
| ˮ | 91.00 | 86.00 |
| Na+ | 0.34 | 0.05 |
| K+ | 0.025 | 0.15 |
| ������ | 0.05 | ���� |
| ���� | 0.00 | 4.60 |
| �������� | 0.06 | 3.70 |
| ������ | 0.002 | ���� |
| �ҵ��� | 0.00 | 2.80 |
| �����ף����壩 | 2.60 | 0.07 |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2013-2014ѧ���㽭ʡ�����и߶�9���¿������Ծ��������棩 ���ͣ��ۺ���
(12��)����������Ƥϸ���ϳ���֭�����Ӫ��������ѪҺ��������ͼΪ��������Ƥϸ���ϳ��������֭�Ĺ���ʾ��ͼ�����ͼ�ش�
(1)�������ҵ������ǵ��л���ļӹ�����������У�����Ҫ���õ�ϸ������_____________����֬����Ҫ�ɷ��Ǹ����������ϳɸ���������ϸ������____________��
(2)ͼ�������ײ�����������Ƥϸ���кϳɣ������������Ƥϸ���ķ�ʽ��____________������Ҫ������ϸ��Ĥ����_______________�Ľṹ�ص㡣�о������ϳɺͷ��ڵ���������һ����õķ�����__________________��
(3)���Ʊ�ϸ��Ĥʱ����ѡ����������Ƥϸ������б�Ƥϸ������ѡ�ò��鶯�����ĺ�ϸ�������׳ɹ���ԭ����______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2014���Ĵ��ϳ���и�����һ���¿�������������棩 ���ͣ��ۺ���
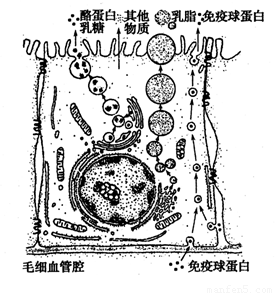
(12��)����������Ƥϸ���ϳ���֭�����Ӫ��������ѪҺ��������ͼΪ��������Ƥϸ���ϳ��������֭�Ĺ���ʾ��ͼ�����ͼ�ش�
(1)�������ҵ������ǵ��л���ļӹ�����������У�����Ҫ���õ�ϸ������_____________����֬����Ҫ�ɷ��Ǹ����������ϳɸ���������ϸ������____________��
(2)ͼ�������ײ�����������Ƥϸ���кϳɣ������������Ƥϸ���ķ�ʽ��____________������Ҫ������ϸ��Ĥ����_______________�Ľṹ�ص㡣�о������ϳɺͷ��ڵ���������һ����õķ�����__________________��
(3)���Ʊ�ϸ��Ĥʱ����ѡ����������Ƥϸ������б�Ƥϸ������ѡ�ò��鶯�����ĺ�ϸ�������׳ɹ���ԭ����______________________________________________________��
(4)��������Ƥϸ�����ܽ��м��������Ե�ϸ���ṹ����ϸ����֮�⣬����______________�����ļ�ϸ����ȣ�������Ƥϸ����___________ (ϸ����)�����϶ࡣ
(5)����ȵ�Bϸ����_________ (����/������)�ϳ������Ļ�����ͼϸ����ϸ������ֲ�����ȥ����ϸ���У����������ɿ�¡����˵��_____________________��
(6)����ͼΪ�˵���������Ƥϸ��,�����ƺ�ƾ�ͨ�� ��ʽ���ս���ϸ����������Υ��ʱ��˾�������������뺬���ظ���ص�����Һ�巴Ӧ����Һ���� ɫ��˵��˾���ƺ��ʻ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2012-2013ѧ���Ϻ�����������㡢�ɽ�����ɽ����������ģ�������Ծ� ���ͣ��ۺ���
�������ϻش������й�ϸ���ṹ�빦�ܵ����⡣��12�֣�
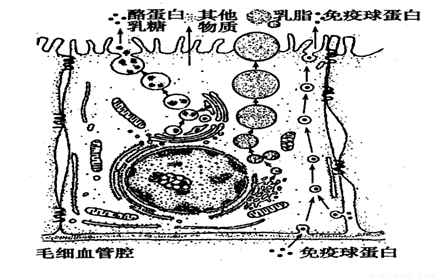
����һ������������Ƥϸ���ϳ���֭�����Ӫ��������ѪҺ��������ͼΪ��������Ƥϸ���ϳ��������֭�Ĺ���ʾ��ͼ�����ͼ�ش�

��1�����ҵ������ǵ��л���ļӹ�����������У�����Ҫ���õ�ϸ������ ����֬����Ҫ�ɷ��Ǹ����������ϳɸ���������ϸ������ ��
��2��ͼ�������ײ�����������Ƥϸ���кϳɣ������������Ƥϸ���ķ�ʽ�� ������Ҫ������ϸ��Ĥ���� �Ĺ��ܡ�
��3�����Ʊ�ϸ��Ĥʱ���Ȳ�ѡ����������Ƥϸ��Ҳ��ѡ����б�Ƥϸ��������ѡ�ò��鶯�����ĺ�ϸ������ԭ���� ������ij�ֻ�ѧҩƷ������ϸ��������ϸ�����ո������������Լ��٣�����������������û��Ӱ�죬˵���ܻ�ѧҩƷӰ����� ��
��4����������Ƥϸ�����ܽ��м��������Ե�ϸ���ṹ����ϸ����֮�⣬���� ��
��5������ȵ�Bϸ���� (���С�������)�ϳ������Ļ���
���϶�����6��������ͼ��֪����DNA���ƿ�ʹ�١� ����ֹͣ��(�����)ϸ���ֻ������Ǣ١� ��(�����)
����������7����ͼ��ijͬѧ���ƵĶ�����ϸ������ģʽͼ�������������Ǵ���ġ��뽫������ȷ��ͼƬ��ϸ������˳�����У��������ڵ�1λ���3λ��ͼƬ�ֱ��� �� ��

�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2011����ͨ�ߵ�ѧУ����ȫ��ͳһ�������������� ���ͣ��ۺ���
������Ƥϸ���ϳ���֭�����Ӫ��������ѪҺ��������Ϊţ������Ƥϸ���ϳ��������֭��ʾ��ͼ���±�Ϊţ��֭��ѪҺ�IJ��ֳɷֱȽϡ�

��1����֭��Ƥϸ���������ڻ�����_____________�����е�K+ ��ͨ��_________��ʽת�˵�������Ƥϸ����
��2����Ѫ����ȣ���֭�����еijɷ���___________________��
��3����֬����Ҫ�ɷ��Ǹ����������ϳɸ���������ϸ������______________________��
��4���ϳ���������������Ҫ����Ѫ�����������ñ���Ѫ����______________________ת��������
��5��ͼ���У�������������Ƥϸ���кϳɵ�����������___________________����ţ�鷿������֢ʱ����������Ѫ������֭�еĺ�����__________�������ʵ�������_________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com