
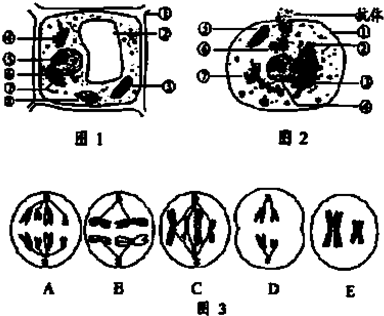
 ���������ڷ��ڵ��ף���ϳɺͷ�������ڹ���Ϊ��������ϳɵ����ʡ����������дּӹ����߶���������ټӹ��γɳ���ĵ����ʡ�ϸ��Ĥ���ɴ˿ɼ�������ĺϳɺͷ��ڹ������ξ���������Ĥ�Ǣ�������Ĥ���߸߶�����Ĥ����ϸ��Ĥ��
���������ڷ��ڵ��ף���ϳɺͷ�������ڹ���Ϊ��������ϳɵ����ʡ����������дּӹ����߶���������ټӹ��γɳ���ĵ����ʡ�ϸ��Ĥ���ɴ˿ɼ�������ĺϳɺͷ��ڹ������ξ���������Ĥ�Ǣ�������Ĥ���߸߶�����Ĥ����ϸ��Ĥ�� �ۢߢ�
�ۢߢ�

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д� �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
| ���� | K+ | M g2+ | Ca2+ | NO3- | H2PO4- | SO42- | Zn2+ |
| ����ҺŨ�ȣ�mol/L�� | 1 | 0.25 | 1 | 2 | 1 | 0.25 | 1 |
| A��Zn2+ |
| B��SO42- |
| C��Ca2+ |
| D��H2PO4- |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
| A������ | B��֬�� | C�������� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�

| T |
| A |
| G |
| C |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
| A���˵�ϸ���еĺ��������֣���DNA��RNA��ǰ����Ҫ������ϸ���ˣ�������Ҫ��ϸ������ |
| B��ʩ���Ǻ�ʩ�����Լ������������ʹ�����һ�η�����ϸ���������ϸ��ѧ˵�� |
| C������һ��������ʢ�������ֲ��ϸ���к�ˮ���������䣬���¶�����ʱ��ϸ��������ˮ�������࣬��л��ǿ |
| D��ϸ���е�Ҷ�����Ⱦɫ�嶼����C��H��O��N��P����Ԫ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com