
(ÿ��1�֣���9��)��ͼ��ʾ֩��˿��ϸ���Ļ���ָ����˿���ϳɹ��̵IJ���ʾ��ͼ�����ͼ�ش�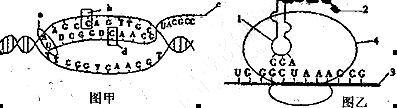
(1)��ͼ��ʾ�Ĺ��̳�Ϊ_______����ͼ�е�b��d�ڻ�ѧ����ϵ�������______��a��һ��ø���ӣ����ܴٽ�c�ĺϳɣ�������Ϊ___________��
(2)��ͼ��1��__________���ù��̵�ģ����____________��
(3)ͼ��4�ṹ������______________�������ж��4��3��ϣ�������2�Ƿ���ͬ��___________________(����ͬ��ͬ)��
(4)����ͼ����Ϣ(UGGGCUAAAGCG)����֪DNAģ�����϶�Ӧ�ļ������Ϊ________________________________________________��
(5)����ͼ���ο��±�������1��Я���İ�������____________________��
| ������ | ������ | �հ��� | ������ | ɫ���� |
| ������ | GCA | ACU | CGU | UGG |
| GCG | ACC | CGC | | |
| GCC | ACA | CGA | | |
| GCU | ACG | CGG | |
��1��ת¼ b��������̼��Ϊ�������ǣ�d��������̼��Ϊ���� RNA�ۺ�ø
��2��tRNA 3��mRNA
��3�������� ��ͬ
��4��ACCCGATTTCGC
��5��������
�������������
��1��������ͼ����ͼ��ʾ��DNA��һ����Ϊģ�壬�ϳ�RNA�Ĺ��̣���ת¼��ͼ��bΪ������������Ǻ����ᣬdΪ����ऺ��Ǻ����ᣬ��ѧ����ϵ�������b�е���̼��Ϊ�������ǣ���d��Ϊ���ǣ�aΪRNA�ۺ�ø��
��2����ͼΪ������̣�ͼ��1ΪtRNA��ת��RNA������������mRNAΪģ����еġ�
��3��ͼ��4Ϊ�����壻���ڷ����ģ����ͬ�����������ϳɵĶ���������ͬ�ġ�
��4����ͼ��mRNA�ļ������ΪUGGGCUAAAGCG�����ݼ���������ԭ��Ӧ��DNAģ�����ļ��������ACCCGATTTCGC��
��5��ͼ����tRNAһ�˵��������ΪCGA����Ϊ�������ӣ��������ӻ�����ԣ����Ӧ��������ΪGCU���������ӱ���֪��Я���İ�����Ϊ�����ᡣ
���㣺���⿼�����ı�����й�֪ʶ�����ڿ��鿼��ʶͼ��������������ѧ֪ʶ��۵㣬ͨ���Ƚϡ��������ۺϵȷ�����ijЩ����ѧ������н��͡������������������жϻ�ó���ȷ�Ľ��۵�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
�о���Ա��ͬ��С�������ij��ҩ�Ƽ����Ļ��أ�һ�ֿ����أ��Ը���֬������Ӱ���ʵ�顣ʵ����ƺͽ�����±���ʾ����ҩ�Ƽ����Ļ��ض���������ˮ�� �⣩����ݱ������ش�
(1)��Ҫ�۲�С��Ƥ��֬��ϸ���е�֬������������______Ⱦɫ��
(2)���н������������������״����ͬ�Ĵ���С���lOOֻ�����ϱ���ʵ����ƣ�Ӧ��ζ���ЩС����з��飿__________________��������ʾ��ʵ�飬�Ա�����______
(3)�����������տ�˵��______���ϱ�ʵ�����ɳ���˵������ҩ�Ƽ��ܶ�С����ʲô���ã�______��
(4)�о���Ա��Ϊ������Ҫ�۲���ҩ�Ƽ���С��û��ע���Ļ��أ�����֬�������� Ӱ�졣������ʵ����Ʋ��ش����⣺
I.ʵ�鲽�裺
�ٲ���һ�����ϱ���ʾ��ʵ����Ƶ�Ҫ������3��С����š�
�ڲ��������3��С���ÿ��ι����ͬ�������⣬ÿ�컹��ֱ�ι______����ll�졣ͬʱ�ڵ�8��ll��ע�����������ˮ����������������������ͬ��
�۲�������������ͳ��ʵ������
II.���ۣ�
�ٽ��õ���ʵ�������ϱ���______��Ľ�����ж��գ�Ȼ��ó����ۡ�
�ڲ��������Ҫ����������������������ͬ������ԭ����______��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ѡ��1-���\��ʵ������15�֣�
��������ֽ���������ǽ����й�����Ӧ��ǰ����ij�о���Ϊ�˴ӳ�����ֲ�������������з�����ܹ���Ч�ֽ���۵�ϸ��������������ʵ�顣���һ������ʵ�����ݲ��ش�������⣺
��1��ʵ�鲽�裺
�������� Ϊ̼Դ�Ĺ�����������Ϊ��ʹ���������̳ɹ��壬Ӧ�����������м���������� ��
�ڽ�������Ʒ���ֵ���������������ϣ��ں���������������һ��ʱ����������ϻ���� ����Ȧ��
�۽��������� ������Ӣ��е�����������ȡһ����ϸ���������� ������塱��������塱��Һ�塱�����������ں���ҡ��������24h��ʹϸ��������ֳ��
��ȡ�������������ͬ�ijɷּ���ȣ�������������Һ��ʹ������������������ɫ�����������PH���� �����۹�������ô�����ֳ��ϸ�����ֵ����������ϡ�
������һ��ʱ��۲������Χ����������ɫ�仯���䷶Χ�Ĵ�С����Χ����
�ľ��伴Ϊ��ѡ���䣬����һ�����롢�����ɴﵽʵ��Ŀ�ġ�
��2������ʵ�鲽���У���ƽ��ͽ��ֵIJ�����Ӧ�ڳ���̨�ϵ� �������С�
��3������ʵ�鲽���У�����;�������������������� ���������ܺ͢��������������� ��������
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
(10��)(2012����̨���)���±���ʾΪij�������������䷽����ش�
| �ɷ� | ���� |
| NaNO3 | 3 g |
| K2HPO4 | 1 g |
| KCl | 0.5 g |
| MgSO4��7H2O | 0.5 g |
| FeSO4 | 0.01 g |
| (CH2O) | 30 g |
| H2O | 1 000 mL |
| ��ù�� | 0.1��λ |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��10�֣���ϸ��������(EPO)�������ڴٽ���ϸ�����ɵ�һ���ǵ��ף�������������˥��ƶѪ�ȼ�����Ŀǰ�ٴ�ʹ�õ��������˺�ϸ��������(rhEPO)�����������������ͼ��ʾ��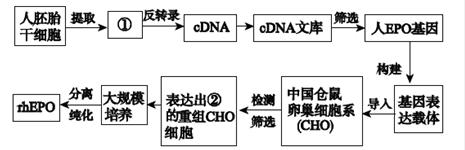
��ش����⣺
(1)ͼ�Т���ָ��������________������ָ��������________��
(2)����������嵼���й������ѳ�ϸ��ϵ(CHO)����õķ�����____________��������������__________��ʶ��ͽ�ϲ�λ��
(3)CHO�Dz�����ѳ�ϸ������ϸ�����������е�___________________������õ�������ֳϸ�������� CHOϸ��������Ҫ____________�Ļ�����Ӫ�����¶ȡ�pH�����廷�����˵�������
(4)���rhEPO������������ÿ�rhEPO�ĵ���¡���塣����¡������ŵ��� �����ɴ����Ʊ���������С��Ĺ�����ϸ�������ض� B�ܰ�ϸ��ͨ��____________������õ��ӽ���ϸ�����ڵġ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��12�֣���ش��ϵ¶��Ŵ����ɼ�����������������Ӧ�õ�������⣺
���㶹�ǽ����Ŵ�ʵ���������ϣ����û�ɫԲ���㶹����ɫԲ���㶹�����ӽ�ʵ��[��ɫ��Y������ɫ��y��Ϊ���ԣ�Բ����R����������r��Ϊ����]�������Ӵ������Ͱ�ÿ�������״����ͳ�ƣ��������ͼ��ʾ����ش����⣺
|

�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��10�֣���ͼ���ұ�ʾѪ�쵰����ȡ�ͷ���IJ���ʵ��װ�ã���ش��������⣺
��1��Ѫ�쵰�����˺������������ϸ������Ҫ��ɳɷ֣����ں�ϸ���е����������˵����ʾ���___________���ܡ�
��2����װ���У�B��Ѫ�쵰����Һ����A��________________����װ���У�C��Һ�������� ______ ��
��3����װ������____________��Ŀ����__________________________������װ�÷��뵰���ʵķ����� ���Ǹ���_______________________________________���뵰���ʵ���Ч������
(4)����װ�÷���Ѫ�쵰��ʱ,������ �����ӽ�ɫ������ʱ,���Թ��ռ�����Һ,ÿ5 mL�ռ�һ��,�����ռ���
��5��Ѫ�쵰����ȡ�ͷ���ij���ɷ�Ϊ�IJ�����Ʒ�������ַ��봿�������ȼ�����������Ʒ�������� �� ���ռ�Ѫ�쵰����Һ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
(7��)��ͼ��ʾϸ����ijЩ�л����Ԫ����ɺ��ܹ�ϵ������A��B����Ԫ�أ������������ӣ�ͼ��X��Y��Z��P�ֱ�Ϊ�����������ӵĻ�����λ����ش��������⣺
(1)ͼ��X��____________������С����������Ҫ��ָ____________��
(2)ͼ��Z��________��ʹ�ü��̡�������ȾҺȾɫ����ʹ�����________ɫ��
(3)ͼ��P�ĽṹͨʽΪ____________��д����P�γɢ��Ľṹ���________________________________________________________________________��
(4)����ϸ���Ļ������У����������������Լ��ٵ���Ҫ��_______ ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
���������ɿ����߲��������һ��ż�㶯�ﴫȾ����Ŀǰ���ý�����������ķ���Ԥ�����������Ҫ�ɷ��Ǹò�����һ�ֽṹ����VP1����ѧ�ҳ�������ת���������������������磬��������ͼ��ʾ�����ͼ�ش�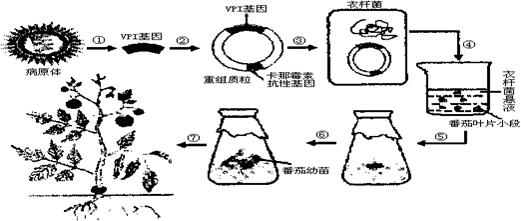
��1�������߲�����VP1�����붯�����ڣ�����������������������Ӧ��VP1���������߷�Ӧ�г�Ϊ ��
��2�������߲������Ŵ�����ΪRNA��Ҫ���VP1������� �ķ����ϳ�DNA������ ��VP1����Ƭ�����¡�
��3�����̢��������г��˼�����ֱ���Ӫ�����ʺ� �ȼ����⣬����Ҫ���� ɸѡ����������������ҶƬС�Ρ�
��4����ñ���VP1���ķ���ֲ���Ժ���Ҫ��������Ч���IJⶨ�����巽���ǣ���ת������ҶƬ��ȡҺע�䵽�������ڣ�ÿ�����ע��һ�Σ����κ�������ѪҺ�ڲ�����
��������Ϊ��ʹ������ţ�Ӧ��������գ��ֱ�ע�� �� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com