
Ņ»Ķć¶¹ŌÓŗĻ×Ó£ØAa£©Ö²Öź×Ō½»Ź±£¬ĻĀĮŠŠšŹö“ķĪóµÄŹĒ
A£®Čō×Ō½»ŗó“ś»łŅņŠĶ±ČĄżŹĒ2:3:1£¬æÉÄÜŹĒŗ¬ÓŠŅžŠŌÅä×ӵĻطŪ50%µÄĖĄĶöŌģ³É
B£®Čō×Ō½»ŗ󓜵ĻłŅņŠĶ±ČĄżŹĒ2:2:1,æÉÄÜŹĒŗ¬ÓŠŅžŠŌÅä×ÓµÄÅßÓŠ50%µÄĖĄĶöŌģ³É
C£®Čō×Ō½»ŗ󓜵ĻłŅņŠĶ±ČĄżŹĒ4:4:1£¬æÉÄÜŹĒŗ¬ÓŠŅžŠŌÅä×ӵēæŗĻĢåÓŠ50%µÄĖĄĶöŌģ³É
D£®Čō×Ō½»ŗ󓜵ĻłŅņŠĶ±ČĄżŹĒ1:2:1£¬æÉÄÜŹĒŗ¬ÓŠŅžŠŌÅä×ӵļ«ŗĖÓŠ50%µÄĖĄ ĶöŌģ³É
ĶöŌģ³É
 æŚĖćĢāæر±¾©ø¾Å®¶łĶƳö°ęÉēĻµĮŠ“š°ø
æŚĖćĢāæر±¾©ø¾Å®¶łĶƳö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2016-2017ѧğŗŚĮś½¹ž¶ū±õĮłÖŠøßŅ»ÉĻĘŚÄ©æ¼ÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
¶ŌČ¾É«ÖŹŗĶČ¾É«ĢåµÄ“ķĪ󊚏öŹĒ£Ø £©
A£®Č¾É«ÖŹŹĒĻø°ūŗĖÄŚŅ×±»¼īŠŌČ¾ĮĻČ¾³ÉÉīÉ«µÄĪļÖŹ
B£®Č¾É«ÖŹŗĶČ¾É«ĢåµÄÖ÷ŅŖ³É·ÖŹĒDNAŗĶµ°°×ÖŹ
C£®Č¾É«ÖŹŗĶČ¾É«ĢåµÄ¹ŲĻµŹĒ²»Ķ¬ÖÖĪļÖŹŌŚĻø°ū²»Ķ¬·ÖĮŃŹ±ĘŚµÄĮ½ÖÖŠĪĢ¬
D£®Č¾É«ÖŹ»ņČ¾É«ĢåÖ»“ęŌŚÓŚÕęŗĖĻø°ūµÄĻø°ūŗĖÖŠ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2017½ģĮÉÄžŹ”ĢśĮėŹŠŠ×÷ĢåøßČżÉĻѧʌµŚĖÄ“ĪĮŖæ¼ÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ijĶ¬Ń§×ܽįĮĖÓŠ¹ŲĻø°ū·ÖĮŃÖŠČ¾É«Ģ唢ŗĖDNA”¢ĖÄ·ÖĢåµÄÖŖŹ¶µć,ĘäÖŠÕżČ·µÄŹĒ£Ø £©
A£®“Ī¼¶¾«ÄøĻø°ūÖŠŗĖDNA·Ö×ÓŹżÄæŹĒÕż³£ĢåĻø°ūÖŠČ¾É«ĢåŹżÄæµÄĮ½±¶
B£®¼õŹżµŚ¶ž“Ī·ÖĮŃŗóĘŚČ¾É«ĢåµÄŹżÄæŹĒÕż ³£ĢåĻø°ūÖŠČ¾É«ĢåŹżÄæµÄŅ»°ė
³£ĢåĻø°ūÖŠČ¾É«ĢåŹżÄæµÄŅ»°ė
C£®³õ¼¶¾«ÄøĻø°ūÖŠČ¾É«ĢåµÄŹżÄæŗĶ“Ī¼¶¾«ÄøĻø°ūÖŠŗĖDNA·Ö×ÓŹżÄæĻąĶ¬
D£®4øöĖÄ·ÖĢåÖŠÓŠ16ĢõČ¾É«Ģå,32øöDNA·Ö×Ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2017½ģĮÉÄžŹ”ĢśĮėŹŠŠ×÷ĢåøßČżÉĻѧʌµŚĖÄ“ĪĮŖæ¼ÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĶ¼±ķŹ¾ČĖĢåÄŚ¾ŽŹÉĻø°ūĶĢŹÉ”¢Ēå³żĖ„ĄĻŗģĻø°ūµÄ¹ż³Ģ”£Ļą¹ŲŠšŹö“ķĪóµÄŹĒ£Ø £©

A£®¢ŚČÜĆøĢ壬ÓÉ¢Ł¶ĻĮŃŗóŠĪ³É£¬ĘäÖŠµÄĆøŅ²Ą“×ŌÓŚ¢Ł
B£®ĶĢŹÉÅŻÓė¢ŚµÄČŚŗĻĢåĻÖĮĖĤµÄĮ÷¶ÆŠŌ
C£®¾ŽŹÉĻø°ūŹ¶±šĖ„ĄĻµÄŗģĻø°ūÓėĻø°ūĤÉĻµÄĢĒµ°°×ÓŠ¹Ų
D£®¢ŚÖŠĖ®½āĆø½«ĶĢŹÉÅŻÖŠµÄµ°°×ÖŹ”¢RNA”¢DNAµČ½µ½ā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2017½ģ½Ī÷ŠĀÓąĖÄÖŠøßČżÉĻѧʌµŚČż“Ī¶Īæ¼ÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ×ŪŗĻĢā
ŅŃÖŖÅŻ²ĖÖŠŃĒĻõĖįŃĪŗ¬ĮæÓėÅŻÖĘŹ±¼äÓŠ¹Ų”£ĪŖĮĖ²ā¶Ø²»Ķ¬ÅŻÖĘĢģŹżÅŻ²ĖÖŠŃĒĻõĖįŃĪµÄŗ¬Į棬ijĶ¬Ń§Éč¼ĘĮĖŅ»øöŹµŃ飬ŹµŃé²ÄĮĻ”¢ŹŌ¼Į¼°ÓĆ¾ß°üĄØ£ŗæĢ¶ČŅĘŅŗ¹Ü”¢±ČÉ«¹Ü”¢²»Ķ¬ÅØ¶ČµÄŃĒĻõĖįÄʱź×¼ČÜŅŗ”¢ŃĒĻõĖįŃĪµÄĻŌÉ«¼Į”¢²»Ķ¬ÅŻÖĘĢģŹżµÄÅŻ²ĖĀĖŅŗµČ”£»Ų“šĻą¹ŲĪŹĢā£ŗ
(1)ĒėĶźÉĘĻĀĮŠŹµŃé²½Öč”£
¢Ł±ź×¼¹ÜµÄÖʱø£ŗÓĆ_______________________ŗĶĻŌÉ«¼ĮÖĘ³ÉŃÕÉ«ÉīĒ³²»Ķ¬µÄĻµĮŠ±ź×¼¹Ü”£
¢ŚŃłĘ·¹ÜµÄÖʱø£ŗÓĆæĢ¶ČŅĘŅŗ¹Ü·Ö±šĪüČ”Ņ»¶ØĮæµÄ_____________________________£¬¼Óµ½²»Ķ¬µÄ±ČÉ«¹ÜÖŠ£¬Č»ŗóŌŚø÷øö±ČÉ«¹ÜÖŠ¼ÓČėµČĮæµÄĻŌÉ«¼Į½ųŠŠĻŌÉ«£¬µĆµ½ŃłĘ·¹Ü”£
¢Ū½«Ćæøö_______·Ö±šÓėĻµĮŠ±ź×¼¹Ü½ųŠŠ±Č½Ļ£¬ÕŅ³öÓėѳʷ¹ÜŃÕÉ«ÉīĒ³_________µÄ±ź×¼¹Ü£¬øĆ¹ÜÖŠŃĒĻõĖįÄĘŗ¬Į漓“ś±ķѳʷ¹ÜÖŠµÄŃĒĻõĖįŃĪŗ¬Į棬¼ĒĀ¼ø÷ѳʷ¹ÜŃĒĻõĖįŃĪµÄŗ¬Į攣
(2)ÅŻ²ĖÖĘ×÷¹ż³ĢÖŠ²śĖįµÄĻø¾śÖ÷ŅŖŹĒ_______________”£
(3)ÅŻ²ĖµÄÖĘ×÷ĄūÓĆĮĖ×ŌČ»½ēÖŠĻֳɵľśÖÖ£¬ČĖĆĒĪŖĮĖ“ļµ½ÄæµÄ»¹ŠčŅŖŌŚŹµŃéŹŅÅąŃųĢŲ¶ØµÄĪ¢ÉśĪļ£¬ĻĀ±ķŹĒÉøŃ”ŅģŃųŠĶĻø¾śµÄÅąŃų»łÅä·½£¬Ēė»Ų“šĻą¹ŲĪŹĢā
KH2PO4 | Na2HPO4 | MgSO4”¤7H2O | FeCl3 | X | Ī¬ÉśĖŲ | ĒķÖ¬ |
1.4 g | 2.1 g | 0.2 g | 0.1 g | 1 g | Ī¢Įæ | 15 g |
¢Ł“ÓĪļĄķŠŌÖŹÉĻæ“øĆÅąŃų»łŹōÓŚ_______ÅąŃų»ł£¬ĘäÖŠ³É·ÖXĪŖÄæµÄ¾śĢį¹©____________”£ÖʱøøĆÅąŃų»łµÄŅ»°ć²Ł×÷Ė³ŠņŹĒ¼ĘĖ攜³ĘĮæ”śČܻƔśĆš¾ś”ś____________”£
¢ŚČēĶ¼A”¢BŹĒ“æ»ÆĪ¢ÉśĪļÅąŃųµÄĮ½ÖÖ½ÓÖÖ·½·Ø£¬C”¢DŹĒ½ÓÖÖŗóÅąŃųµÄŠ§¹ū”£Ä³Ķ¬Ń§½ÓÖÖÅąŃųŗó»ńµĆĶ¼CĖłŹ¾Š§¹ū£¬ŌņĘä²ÉÓĆµÄ½ÓÖÖ·½·ØŹĒ[””””]______________________([””””]Ń”Ģī”°A”±”°B”±)£»½ÓÖÖĒ°ŠčŅŖ¼ģ²āÅąŃų»łŹĒ·ń±»ĪŪČ¾£¬¼ģ²ā·½·ØŹĒ½«Ī“½ÓÖÖµÄÅąŃų»łŌŚŹŹŅĖĪĀ¶ČĻĀÅąŃų£¬¹Ū²ģ____________”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2017½ģ½Ī÷ŠĀÓąĖÄÖŠøßČżÉĻѧʌµŚČż“Ī¶Īæ¼ÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
Ļø°ūĖł“¦µÄÄÜĮæדĢ¬ÓĆATP”¢ADPŗĶAMPÖ®¼äµÄ¹ŲĻµŹ½Ą“±ķŹ¾£¬³ĘĪŖÄÜŗÉ£¬¹«Ź½ČēĻĀ:ÄÜŗÉ= £¬ĘäÖŠAMPĪŖŅ»Į×ĖįĻŁÜÕ”£ÄÜŗɶŌ“śŠ»Ęš×ÅÖŲŅŖµÄµ÷½Ś×÷ÓĆ£¬øßÄÜŗÉŹ±£¬ATPÉś³É¹ż³Ģ±»ŅÖÖĘ£¬¶ųATPµÄĄūÓĆ¹ż³Ģ±»¼¤·¢£»µĶÄÜŗÉŹ±£¬Ę䊧ӦĻą·“”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø £©
£¬ĘäÖŠAMPĪŖŅ»Į×ĖįĻŁÜÕ”£ÄÜŗɶŌ“śŠ»Ęš×ÅÖŲŅŖµÄµ÷½Ś×÷ÓĆ£¬øßÄÜŗÉŹ±£¬ATPÉś³É¹ż³Ģ±»ŅÖÖĘ£¬¶ųATPµÄĄūÓĆ¹ż³Ģ±»¼¤·¢£»µĶÄÜŗÉŹ±£¬Ę䊧ӦĻą·“”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø £©
A£®øł¾ŻÄÜŗɵĹ«Ź½×é³É£¬ĶĘ²āŅ»°ćĒéæöĻĀĻø°ūÄÜŗÉŹżÖµŠ”ÓŚ1
B£®Ļø°ūÖŠATP”¢ADPŗĶAMPÖ®¼äæÉŅŌĻą»„×Ŗ»Æ
C£®Ļø°ūŌŚĪüŹÕMg2+Ź±£¬ÄÜŗɽĻµĶ
D£®ÄÜŗɼ°Ęäµ÷½ŚŹĒÉśĪļ½ēµÄ¹²ŠŌ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2017½ģŗÓÄĻŹ”øßČżÉĻѧʌ¼ģ²āĮ·Ļ°ÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĒšÄŌŹĒĪ»ÓŚ“óÄŌʤ²ćø¹Ćęµ÷½ŚÄŚŌą»ī¶ÆµÄøß¼¶ÖŠŹą£¬µ÷½Ś×ÅĢåĪĀ”¢Ė®Ę½ŗā”¢ŃŖĢĒŗĶÄŚ·ÖĆŚĻŁ»ī¶ÆµČÖŲŅŖµÄÉśĄķ¹¦ÄÜ£®ĻĀĮŠĻą¹ŲŠšŹöÕżČ·µÄŹĒ
A£®ČōÖ»ŅŖĘĘ»µĻĀĒšÄŌ£¬ŹµŃé¶ÆĪļ¾Ķ²»¾ßÓŠĪ¬³ÖĢåĪĀŗć¶ØµÄÄÜĮ¦£¬±ķĆ÷µ÷½ŚĢåĪĀµÄÖ÷ŅŖÖŠŹąŌŚĻĀĒšÄŌ
B£®µ±ŃŖĢĒÅضČÉĻÉżŹ±£¬ĻĀĒšÄŌÖŠµÄĘĻĢŃĢĒøŠŹÜĘ÷½ÓŹÜ“Ģ¼¤²śÉśŠĖ·Ü£¬Ź¹ŅȵŗBĻø°ūŗĻ³É·ÖĆŚŅȵŗĖŲ¼õÉŁ
C£®ĻĀĒšÄŌĖš»µŗó£¬ŹµŃé¶ÆĪļ·ÖĆŚµÄæ¹ĄūÄņ¼¤ĖŲ¼õÉŁ£¬ÖĀŹ¹ÉöŠ”¹ÜŗĶ¼ÆŗĻ¹ÜÖŲĪüŹÕĖ®·Ö¼õÉŁ£¬æɵ¼ÖĀŹµŃé¶ÆĪļ·³æŹÓėÉŁÄņ
D£®Ä³Š©ÓÉÓŚŃŖŅŗÖŠŗ¬ÓŠæ¹ŅȵŗBĻø°ūµÄæ¹ĢåŗĶŠ§Ó¦TĻø°ūµÄĢĒÄņ²”»¼Õߣ¬²»ÄÜĶعż×¢ÉäŅȵŗĖŲĄ“»ŗ½ā²”Ēé
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2017½ģŗÓÄĻŹ”øßČżÉĻѧʌ¼ģ²āĮ·Ļ°ÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĪøÄŚµÄĖįŠŌ»·¾³ŹĒĶعżÖŹ×Ó±ĆĪ¬³ÖµÄ£¬ÖŹ×ӱƓ߻Æ1·Ö×ÓµÄATPĖ®½āĖłŹĶ·ÅµÄÄÜĮ棬æÉĒż¶Æ1øöH+“ÓĪø±ŚĻø°ū½ųČėĪøĒ»ŗĶ1øöK+“ÓĪøĒ»½ųČėĪø±ŚĻø°ū£¬K+ÓÖæɾĶصĄµ°°×Ė³ÅØ¶Č½ųČėĪøĒ»”£ĻĀĮŠĻą¹ŲŠšŹö“ķĪóµÄŹĒ
A. ÖŹ×ӱƵĻł±¾×é³Éµ„Ī»æÉÄÜŹĒ°±»łĖį
B. H+“ÓĪø±ŚĻø°ū½ųČĖĪøĒ»ŠčŅŖŌŲĢåµ°×Ō
C. Īø±ŚĻø°ūÄŚK+µÄŗ¬ĮæÓ°ĻģĻø°ūÄŚŅŗÉųĶøŃ¹µÄ“óŠ”
D. K+½ų³öĪø±ŚĻø°ūµÄæēĤŌĖŹä·½Ź½ŹĒĻąĶ¬µÄ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2017½ģŗÓÄĻŹ”øßČżÉĻѧʌ12.4ÖÜæ¼ÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
Ķ¼¼×±ķŹ¾ĖÄÖÖ²»Ķ¬µÄĪļÖŹŌŚŅ»øö¶ÆĪļĻø°ūÄŚĶāµÄĻą¶ŌÅØ¶Č²īŅģ”£ĘäÖŠĶعżĶ¼ŅŅĖłŹ¾µÄ¹ż³ĢĄ“Ī¬³ÖĻø°ūÄŚĶāÅØ¶Č²īŅģµÄĪļÖŹŹĒ
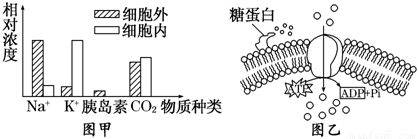
A. Na+ B. K+ C. ŅȵŗĖŲ D. CO2
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com