
£Ø8·Ö£©½«ĶÜÄŌĘĘ»µ£¬±£Įō¼¹Ėč£¬×öĶ܊ľ²Āö¹ą×¢£¬ŅŌĪ¬³ÖĶܵĻł±¾ÉśĆü»ī¶Æ”£±©Ā¶ĶÜ×óŗóÖ«Ēü·“ÉäµÄ“«ČėÉń¾ŗĶ“«³öÉń¾£¬·Ö±šĮ¬½ÓµēĪ»¼ĘaŗĶb”£½«ĶÜ×óŗóÖ«Öŗ¼ā½žČė0.5%ĮņĖįČÜŅŗŗ󣬵ēĪ»¼ĘaŗĶbÓŠµēĪ»²Ø¶Æ£¬³öĻÖĒü·“É䔣ĻĀĶ¼ĪŖøĆ·“Éä»”½į¹¹Ź¾ŅāĶ¼”£
£Ø1£©ÓĆ¼ņ±ćµÄŹµŃéŃéÖ¤ŠĖ·ÜÄÜŌŚÉń¾ĻĖĪ¬ÉĻĖ«Ļņ“«µ¼£¬¶ųŌŚ·“Éä»”ÖŠÖ»Äܵ„Ļņ“«µŻ£¬Š“³ö·½·ØŗĶĻÖĻ󔣓Ģ¼¤__________Ö®¼äµÄÉń¾, ¹Ū²ģµ½µēĪ»¼ĘbÓŠµēĪ»²Ø¶ÆŗĶ×óŗóÖ«ĒüĶČ£¬µēĪ»¼Ęa________________£Ø³öĻÖ»ņĪ“³öĻÖ£©µēĪ»²Ø¶Æ”£
£Ø2£©ČōŌŚ¹ą×¢ŅŗÖŠĢķ¼ÓijÖÖŅ©Īļ£¬½«ĶÜ×óŗóÖ«Öŗ¼ā½žČė0.5%ĮņĖįČÜŅŗŗ󣬵ēĪ»¼ĘaÓŠ²Ø¶Æ£¬µēĪ»¼ĘbĪ“³öĻֲضƣ¬×óŗóÖ«Ī“³öĻÖĒü·“É䣬ĘäŌŅņæÉÄÜÓŠ£ŗ¢Ł £»¢Ś ”£
¢Å·½·ØŗĶĻÖĻó£ŗ“Ģ¼¤µēĪ»¼ĘbÓė¹Ē÷Ą¼”Ö®¼äµÄ“«³öÉń¾”£¹Ū²ģµ½µēĪ»¼ĘbÓŠµēĪ»²Ø¶ÆŗĶ×óŗóÖ«ĒüĶČ£¬µēĪ»¼ĘaĪ“³öĻÖµēĪ»²Ø¶Æ ¢ĘĶ»“„Ē°Ä¤ŹĶ·ÅµÄµŻÖŹ²»ÄÜÓėĶ»“„ŗóĤÉĻµÄĢŲŅģŠŌŹÜĢå½įŗĻ Ķ»“„Ē°Ä¤²»ÄÜŹĶ·ÅµŻÖŹ
½āĪöŹŌĢā·ÖĪö£ŗ¢Å“Ģ¼¤µēĪ»¼ĘbÓė¹Ē÷Ą¼”Ö®¼äµÄ“«³öÉń¾”£¹Ē÷Ą¼”ŹÕĖõĒŅµēĮ÷±ķb·¢ÉśĘ«×Ŗ£¬ĖµĆ÷Éń¾ĻĖĪ¬ŹÜ“Ģ¼¤²śÉśµÄ³å¶ÆŌŚÉń¾ĻĖĪ¬ÉĻŹĒĖ«Ļņ“«µ¼£¬µ«ÓÉÓŚµēĮ÷±ķa²»Ę«×Ŗ£¬ĖµĆ÷Éń¾³å¶Æ²»ÄÜ“ÓĶ»“„ŗóĤ“«ÖĮĶ»“„Ē°Ä¤”£¢Ę½«ĶÜ×óŗóÖ«Öŗ¼ā½žČė0.5%ĮņĖįČÜŅŗ£¬£¬µēĪ»¼ĘaÓŠ²Ø¶Æ£¬µēĪ»¼ĘbĪ“³öĻֲضƣ¬×óŗóÖ«Ī“³öĻÖĒü·“É䣬ĖµĆ÷ŠĖ·Üƻӊ“ÓĶ»“„Ē°Ä¤“«µŻµ½Ķ»“„ŗóĤ£¬Ņņ“ĖŌŅņæÉÄÜÓŠ£ŗĶ»“„Ē°Ä¤ŹĶ·ÅµÄµŻÖŹ²»ÄÜÓėĶ»“„ŗóĤÉĻµÄĢŲŅģŠŌŹÜĢå½įŗĻ»ņÕßĶ»“„Ē°Ä¤²»ÄÜŹĶ·ÅµŻÖŹ”£
æ¼µć£ŗ±¾ĢāÖ÷ŅŖ漲鷓Éä»”µÄ½į¹¹£¬ŅāŌŚæ¼²éæ¼ÉśÄÜĄķ½āĖłŃ§ÖŖŹ¶µÄŅŖµć£¬°ŃĪÕÖŖŹ¶¼äµÄÄŚŌŚĮŖĻµ£¬ŠĪ³ÉÖŖŹ¶µÄĶųĀē½į¹¹µÄÄÜĮ¦”£


 ÓÅѧĆūŹ¦ĆūĢāĻµĮŠ“š°ø
ÓÅѧĆūŹ¦ĆūĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
£Ø10·Ö£©Ä³ÖÖĆø½ųŠŠÓŠ¹ŲŹµŃéµÄ½į¹ūČēĻĀĶ¼ĖłŹ¾£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©¾ŻĶ¼Ņ»”¢Ķ¼¶žÅŠ¶Ļ£¬øĆĆøµÄ×īŹŹ·“Ó¦Ģõ¼žŹĒ ”£
£Ø2£©Ķ¼Ņ»ÖŠAµćÉś³ÉĪļµÄĮæ²»ŌŁŌö¼ÓµÄŌŅņŹĒ ”£
£Ø3£©Ķ¼ČżÖŠ£¬µ×ĪļÅضČĪŖBŹ±£¬Ó°ĻģĆø»īŠŌµÄĶā½ēŅņĖŲŹĒ ”£
£Ø4£©“ÓĶ¼ĖÄæÉÖŖ£¬øĆĆø×÷ÓƵĵ×ĪļŹĒ £¬ĖµĆ÷ĆøµÄ×÷ÓĆ¾ßÓŠ ŠŌ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
ÅŠ¶ĻĢā£Ø8·Ö£©£ŗ
¢ŁĢåŅŗŹĒÖøĻø°ūĶāŅŗ”££Ø £©
¢ŚĢåÄŚĻø°ūĶعżÄŚ»·¾³æÉŅŌÓėĶā½ē»·¾³½ųŠŠĪļÖŹ½»»»”££Ø £©
¢Ū¶ĢĘŚ¼ĒŅäÖŲø“æÉ×Ŗ»ÆĪŖ³¤ĘŚ¼ĒŅ䔣£Ø £©
¢ÜĆāŅßĻµĶ³¼ČŹĒ»śĢåµÄ·ĄÓłĻµĶ³£¬Ņ²ŹĒĪ¬³ÖĪČĢ¬µÄµ÷½ŚĻµĶ³”££Ø £©
¢ŻÄŚ»·¾³ĪČĢ¬ŹĒ»śĢå½ųŠŠÕż³£ÉśĆü»ī¶ÆµÄ±ŲŅŖĢõ¼ž”££Ø £©
¢ŽČĖĢåĪ¬³ÖĪČĢ¬µÄµ÷½ŚÄÜĮ¦ŹĒÓŠŅ»¶ØµÄĻŽ¶ČµÄ”££Ø £©
¢ßø÷øöÉń¾ÖŠŹą¼äŹĒĻą»„ĮŖĻµ”¢Ļą»„µ÷æŲµÄ”££Ø £©
¢ąÄŚ»·¾³ĪČĢ¬ŹĒÖøÄŚ»·¾³µÄ³É·ÖŗĶĄķ»ÆŠŌÖŹŗć¶Ø²»±ä”££Ø £©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
£Ø10·Ö£©ģ³ĮÖŹŌ¼ĮµÄ¼×ŅŗŹĒÖŹĮæÅضČĪŖ0.1 g/mLµÄNaOHČÜŅŗ£¬ŅŅŅŗŹĒÖŹĮæÅضČĪŖ0.05 g/mLµÄCuSO4ČÜŅŗ”£Ė«ĖõėåŹŌ¼ĮµÄAŅŗŹĒÖŹĮæÅضČĪŖ0.1 g/mLµÄNaOHČÜŅŗ£¬BŅŗŹĒÖŹĮæÅضČĪŖ0.01 g/mLµÄCuSO4ČÜŅŗ”£Ä³Ķ¬Ń§ŌŚ”°¼ģ²āÉśĪļ×éÖÆÖŠµÄĢĒĄą”¢Ö¬·¾ŗĶµ°°×ÖŹ”±µÄŹµŃéÖŠ£¬²śÉśĮĖŅ»Š©ŅÉĪŹ£¬ŌŚÕ÷µĆĄĻŹ¦Ķ¬Ņāŗó£¬ĖūĄūÓĆæĪĶāŹ±¼ä½ųŠŠĮĖĢ½¾æ”£ĻĀ±ķŹĒĖūµÄĢ½¾æŹµŃéµÄ²æ·Ö²Ł×÷£ŗ
| ŹŌ¹Ü | µŚ1“Ī¼ÓČėĪļ | µŚ2“Ī¼ÓČėĪļ | µŚ3“Ī¼ÓČėĪļ |
| 1ŗÅ | 1 mL 0.1 g/mL NaOHČÜŅŗ | 1 mL 0.05 g/mL CuSO4ČÜŅŗ | 2 mL Ę»¹ūÖ |
| 2ŗÅ | 1 mL 0.1 g/mL NaOHČÜŅŗ | 2 mLĘ»¹ūÖ | 1 mL 0.05 g/mL CuSO4ČÜŅŗ |
| 3ŗÅ | 2 mLĘ»¹ūÖ | 1 mL 0.1 g/mL NaOHČÜŅŗ | 1 mL 0.05 g/mL CuSO4ČÜŅŗ |
| 4ŗÅ | 1 mL 0.05 g/mL CuSO4ČÜŅŗ | 2 mLĘ»¹ūÖ | 1 mL 0.1 g/mL NaOHČÜŅŗ |
| 5ŗÅ | 1 mL 0.1 g/mL NaOHČÜŅŗ | 2 mLĘ»¹ūÖ | 4µĪ0.01 g/mL CuSO4ČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
£Ø9·Ö£©Ä³Ķ¬Ń§ĪŖĮĖĢ½¾æ²»Ķ¬Ģõ¼ž¶ŌÖ²Īļ¹āŗĻ×÷ÓĆĖŁĀŹŗĶŗōĪü×÷ÓĆĖŁĀŹµÄÓ°Ļģ£¬×öĮĖČēĻĀŹµŃé£ŗÓĆ8Öźø÷ÓŠ20ʬŅ¶Ę¬”¢“óŠ”ŗĶ³¤ŹĘĻąĖʵÄĢģóĆæūÅčŌŌÖ²Öź£¬·Ö±š·ÅŌŚĆܱյIJ£Į§ČŻĘ÷ÖŠ£¬ŌŚ²»Ķ¬ŹµŃéĢõ¼žĻĀ£¬ĄūÓĆ“«øŠĘ÷¶ØŹ±²ā¶ØĆܱÕČŻĘ÷ÖŠ¶žŃõ»ÆĢ¼ŗ¬ĮæµÄ±ä»Æ”£ŹµŃé½į¹ūĶ³¼ĘČēĻĀ±ķ£¬Ēė·ÖĪö»Ų“šĻĀĮŠĪŹĢā£ŗ
| ±ąŗÅ | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| ĪĀ¶Č£Ø”ę£© | 10 | 10 | 20 | 20 | 30 | 30 | 40 | 40 |
| ¹āÕÕĒæ¶Č£ØLx£© | 1000 | 0 | 1000 | 0 | 1000 | 0 | 1000 | 0 |
| æŖŹ¼Ź±CO2Įæ£Øg£© | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| 12£čŗóCO2Įæ£Øg£© | 4.5 | 5.1 | 3.5 | 5.4 | 1.9 | 5.9 | 2.0 | 5.8 |
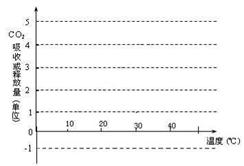
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
£Ø8·Ö£©ČēĶ¼ŹĒijũŅµÉśĢ¬ĻµĶ³µÄÄ£Ź½Ķ¼”£Ēė¾ŻĶ¼»Ų“šĻĀĮŠĪŹĢā£ŗ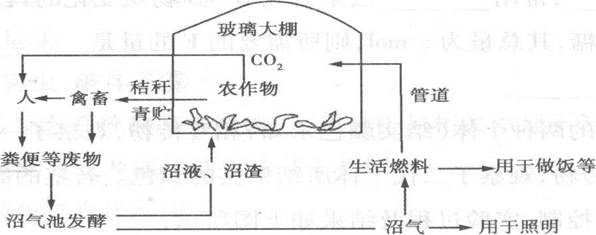
(1)øĆÉśĢ¬ĻµĶ³µÄ³É·ÖÓŠ______________£¬Ļą¶ŌŅ»°ćÅ©ŅµÉśĢ¬ĻµĶ³¶ųŃŌ£¬“ĖÉśĢ¬ĻµĶ³ÄÜĢįøß¾¼ĆŠ§Ņę£¬Ö÷ŅŖŌŅņŹĒ³ä·Ö ĄūÓĆĮĖ______________ÖŠµÄÄÜĮ攣
(2)ÓÉÓŚ×ŌČ»ŌÖŗ¦µÄ·¢Éś£¬øĆ“åĀäÓėĶā½ēŹ§Č„ĮĖĶØѶĮŖĻµ£¬²»Äܼ°Ź±µĆµ½Īļ׏¹©Ó¦£¬ÓČĘäŹĒĮøŹ³½ōȱ£¬Čō“Ė“åĀä ŌŚ½Ļ³¤Ņ»¶ĪŹ±¼äÄŚ²»ÄܵƵ½Ķā½ēŌ®ÖśĒŅÉŠæÉÅ©øū¼°ŠóÄĮŃųÖ³£¬“ĖÖÖדĢ¬ĻĀæÉŅŌŹŹµ±øıäÉÅŹ³½į¹¹”£ĄżČē£¬ Čō½«£Ø²ŻŹ³£©¶ÆĪļŠŌÓėÖ²ĪļŠŌŹ³ĪļµÄ±ČĄżÓÉ1£ŗ1µ÷ÕūĪŖ1£ŗ4£¬ŌņĻÖÓŠČĖČŗæÉ“ę»īµÄŹ±¼ä½«ŹĒŌĄ“µÄ__________ ±¶£ØÄÜĮæ“«µŻŠ§ĀŹ°“10%¼ĘĖć£¬½į¹ū¾«Č·µ½Š”ŹżµćŗóĮ½Ī»Źż×Ö£©”£
(3)ĒėÓĆ¼żĶ·ŗĶĪÄ×Ö±ķŹ¾ÕāøöÉśĢ¬ĻµĶ³ÖŠÄÜĮæĮ÷¶ÆµÄĶ¼½ā£Øø÷ÖÖÉśĪļµÄŗōĪüŗÄÄܲ»ŅŖĒó»³ö£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
£Ø6·Ö£©Ķ¼¼×±ķŹ¾Ö²ĪļĻø°ū“śŠ»µÄijŠ©¹ż³Ģ£¬Ķ¼ŅŅ±ķŹ¾¹āÕÕĒæ¶ČÓė¶žŃõ»ÆĢ¼±ä»ÆĮæµÄ¹ŲĻµ”£Ēė¾ŻĶ¼»Ų“šĪŹĢā”££ØĶ¼ÖŠŹż×Ö“ś±ķĪļÖŹ£¬a”¢b”¢c“ś±ķĻø°ūĘ÷£©
£Ø1£©Ķ¼¼×ÖŠ£¬Ļø°ūĘ÷b”¢c·Ö±šĪŖ ”£
£Ø2£©Ķ¼¼×ÖŠĪļÖŹ¢ŪŹĒ___________£¬Ęä½ųČėcÖŠ±»·Ö½ā”£Ó°Ļģb”¢cÖŠÉśĄķ¹ż³ĢµÄ¹²Ķ¬µÄ»·¾³ŅņĖŲÖ÷ŅŖŹĒ ”£
£Ø3£©½«Ņ»ÖźÖ²Īļ·ÅÖĆÓŚĆܱյÄČŻĘ÷ÖŠ£¬ÓĆŗģĶā²āĮæŅĒ½ųŠŠ²āĮ棬²āĮæŹ±¼ä¾łĪŖ1Š”Ź±£¬²ā¶ØµÄĢõ¼žŗĶ½į¹ūČēÉĻĶ¼ŅŅĖłŹ¾£¬£ØŹż¾Ż¾łŌŚ±ź×¼×“æöĻĀ²āµÄ£©¾Ż“Ė»Ų“š£ŗČōøĆÖ²ĪļŌŚ³ä·Ö¹āÕÕĻĀ»żĄŪµÄÓŠ»śĪļ¶¼ŹĒĘĻĢŃĢĒ£¬ŌŚ25”ę”¢4Ē§ĄÕæĖĖ¾¹āÕÕĢõ¼žĻĀ£¬øĆÖ²ĪļŌŚ³ä·Ö¹āÕÕĻĀ1Š”Ź±×ܹ²»żĄŪĘĻĢŃĢĒ ŗĮæĖ”£
£Ø4£©“ÓĶ¼ŅŅÖŠæÉ·¢ĻÖ£¬Ó°ĻģAµć¹āŗĻĖŁĀŹµÄŅņĖŲŹĒ ”£
£Ø5£©ČōøųøĆĆܱÕ×°ÖĆĶØČė £¬Ņ»¶ĪŹ±¼äŗó£¬×°ÖĆÄŚ³öĻÖĮĖ·ÅÉäŠŌµÄĘųĢåÓŠ__________”£
£¬Ņ»¶ĪŹ±¼äŗó£¬×°ÖĆÄŚ³öĻÖĮĖ·ÅÉäŠŌµÄĘųĢåÓŠ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
øÆČ锢ėē°×²ĖµČŅŌĘäÉ«ŌóÓÕČĖ”¢Ī¶µĄĻŹĆĄŹ±³£³öĻÖŌŚČĖĆĒµÄ²Ķץ£¬Õā¼øÖÖŹ³Ę·¶¼ŹĒ¾¹żėēÖĘ¼Ó¹¤¶ų³ÉµÄ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ

£Ø1£©øÆČéÖĘ×÷¹ż³ĢÖŠ£¬¶ąÖÖĪ¢ÉśĪļ²ĪÓė·¢½Ķ×÷ÓĆ£¬ĘäÖŠĘšÖ÷ŅŖ×÷ÓƵÄĪ¢ÉśĪļŹĒŅ»ÖÖ______דÕę¾ś”£Ķ¼ÖŠA±ķŹ¾ £¬ÖĘ×÷øÆČéÅ÷Ź±ÓĆ·ŠĖ®“¦Ąķ¶¹øƵÄÄæµÄŹĒ £¬¶¹øÆŗ¬Ė®Įæ²»ÄܹżøßŌŅņŹĒ ”£
£Ø2£©øÆČéÖĘ×÷¹ż³ĢÖŠ¼ÓŃĪµÄ×÷ÓĆŹĒ___ _____ŗĶ__ ___”£
£Ø3£©ÖĘ×÷øÆČéµÄĀ±ĢĄÖŠĶس£ŅŖ¼ÓČėĮĻ¾Ę”¢»Ę¾ĘµČ£¬ŗ¬ĮæŅ»°ćæŲÖĘŌŚ12£„×óÓŅ£¬ĘäÄæµÄŹĒ ”£
£Ø4£©ÅŻ²Ė·¢½Ķ¹ż³ĢÖŠ£¬»į²śÉś¶ąÖÖĖį£¬ĘäÖŠÖ÷ŅŖŹĒČéĖį£¬»¹ÓŠÉŁĮæµÄŃĒĻõĖį”£¶ŌŃĒĻõĖįŃĪµÄ¶ØĮæ²ā¶ØæÉŅŌÓĆ ·Ø£¬ŅņĪŖŃĒĻõĖįŃĪÓė¶Ō°±»ł±½»ĒĖįµÄ·“Ó¦²śĪļÄÜÓėN-1-ŻĮ»łŅŅ¶ž°·ŃĪĖįŃĪżĮŖ³É É«»ÆŗĻĪļ”£
£Ø5£©¾ŻĶ¼æÉÖŖėē°×²ĖŌŚėēÖĘ_________ĢģŗóŹ³ÓĆ×ī¼Ń”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
£Ø8·Ö£©¼×”¢ŅŅĮ½ÖÖ»ÆѧĪļÖŹæÉÄÜÓŠŅ»¶ØÖĀ»ū”¢ÖĀ°©×÷ÓĆ£¬ĄūÓƶÆĪļĻø°ūÅąŃųµÄ·½·Ø¼ų¶ØĖüĆĒŹĒ·ńÓŠ¶¾ŠŌ£¬²¢±Č½Ļ¶žÕ߶¾ŠŌµÄĒæČõ”£ĒėŅĄ¾ŻŹµŃé½į¹ūĶź³ÉĻĀĮŠŹµŃé±Øøę”£
I£®ŹµŃé²½Öč£ŗ
£Ø1£©ÖʱøĻø°ūŠüŅŗ£ŗÓĆ “¦ĄķŠ”°×ŹóÅßĢ„×éÖÆĄėÉ¢³Éµ„øöĻø°ū£¬ÖĘ³ÉĻø°ūŠüø”Ņŗ”£
£Ø2£©½ųŠŠĻø°ūÅąŃų£ŗ
¢Ł Č”A£®B£®CČżøö½ą¾»µÄ׶ŠĪĘ棬·Ö±š¼ÓČė ”£
¢Ś ĻņA£®BĮ½øöÅąŃųĘæÖŠ·Ö±š¼ÓČėµČĮæµÄ»ÆѧĪļÖŹ¼×”¢ŅŅ£¬²¢½«ÅąŃųĘæŅ”ŌČ£»
CĘæ²»×÷“¦Ąķ£¬×÷ĪŖ¶ŌÕÕ”£
¢Ū ”££ØŅŖĒ󊓳öÅąŃų³”Ėł£©”£
£Ø2£©ÖĘ×÷ĮŁŹ±×°Ę¬
£Ø3£©¾µ¼ģŗĶĶ³¼Ę£ŗ°ŃĮŁŹ±×°Ę¬·ÅŌŚĻŌĪ¢¾µĻĀ£¬Ń°ÕŅ“¦ÓŚ ĘŚµÄĻø°ū£¬ÓėŠ”°×ŹóÕż³£ĢåĻø°ūÓŠĖæ·ÖĮŃøß±¶ĻŌĪ¢¾µÕÕʬ½ųŠŠ¶Ō±Č£¬ŅŌČ·ČĻ·¢Éś±äŅģµÄĻø°ū”£Ķ³¼Ę ”£
¢ņ£®ŹµŃé½į¹ū£ŗ
| ÅąŃųĘæ | A | B | C |
| ±äŅģĻø°ūÕ¼×ÜĻø°ūŹż | 1.3% | 12.5% | 0.1% |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com