
�Ķ����²��ϻش��������⣺
�����ҹ���ȫ����������H1N1�����������еĹ��ҡ�Ŀǰ����������ʳƷҩƷ�ල�������������еļ���H1N1��������Ϊ15���������ѽ����磬ע����͡�����Ŀǰ�������е�������8����ҵ�����������л���������������˾������֮һ�������ļ���H1N1���������������������֯�Ƽ��ļ���H1N1���в����꣬�������������������ļ������������������ͬ�����辭�������������������������ѽ�ȹ��պ��Ƴɡ������Ʊ���������������ͼ���H1N1��������Ĺ��̣����輦�����������ѽ⡢�����ȹ��գ������в��ɱ���Ļ�����������嵰�ס���ȩ���ѽ�������ʣ���˶������κγɷֹ�������Ӧ��ֹ���ּ���H1N1�������硣��ʵ�ϣ����й��ļ�������Ʒ�ֶ��ԣ���Ϊ�������磬�������Ǵ���ģ������ǡ������ģ���û�ж��Եģ���ˣ�Ҳ��������Ϊ�����еIJ�����ֳ���ƣ������»�������������������ϵ��
���������������Ҫ�ɷ��Ǿ����ᴿ�ķ��������Ĥ���ǣ����ַ�������ֻ��Ԥ���ɷ����������ĸ�Ⱦ������Ԥ������H1N1���в�����������С�
����H1N1���в�����________��ϸ������ϸ��������������ܶ����³´�л�������ڻ�ϸ���ڲ�����ֳ������ע���H1N1����������ѧ�ϳ�Ϊ________���������������ϸ������ȡ�ʹ�������¶�����ֲ�ԭ�����еĿ�ԭ������ԭ��Ϣ�ʵݸ�Tϸ�����̼�Tϸ������________������________ֱ�Ӵ̼�Bϸ����Bϸ���ܵ��̼������ܰ����ӵ������£���һϵ�еķֻ���ֳ�γ�________������������Ӧ��________��С�����γ�________ϸ����________����������H1N1������ϣ��������Ʋ����ķ�ֳ�������ϸ����𤸽���Ӷ���ֹ��Ⱦ����H1N1���кͼ����ķ�����
������������������ߣ��Լ���H1N1���е��������ڵ�________�����ߣ���Ϊ________�����ַ�������ֻ��Ԥ���ɷ����������ĸ�Ⱦ������Ԥ������H1N1���в�����������С�
 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ����������� ���ͣ�022
A�����Ἣ���ȶ����ڽ�Ϊ���ҵĻ�ѧ���������غ�ø�������º������⡣���Ʊ�DNAʱҪ����DNAø��ˮ��DNA��ø�������Ƽ��������ƣ��Գ�ȥMg����ֹDNAø�ļ��
B�������е�DNA��RNA���������ھ��Ժ˵��ף��ɺ���͵�������ɣ�����ʽ���ڣ�DNA�˵�����1mol![]() NaCl��Һ���ܽ�Ⱥܴ���0.14mol��
NaCl��Һ���ܽ�Ⱥܴ���0.14mol��![]() NaCl��Һ�е��ܽ�Ⱥܵͣ���RNA�˵�������0.14mol��
NaCl��Һ�е��ܽ�Ⱥܵͣ���RNA�˵�������0.14mol��![]() NaCl��Һ��
NaCl��Һ��
C�������Ӵ�������ʹ�����ʱ��ԣ������ڱ��Ӳ��ڣ���DNA��Һ�м���2.5�������Ũ��Ϊ95���ľƾ����ɽ�DNA�����������ʱDNAʮ��ճ�������ò���������ȡ����
D��DNA��ǿ�ỷ���£�ˮ������������ǵ�С�������ʣ����������������Һ��Ӧ����������ɫ�����
E��ʵ���������е������������Һ��ʯӢɰ��0.14mol��![]() NaCl��Һ��1mol��
NaCl��Һ��1mol��![]() NaCl��Һ������ˮ�����ӡ�95���ƾ����������Լ���Ũ���ᡢ��Ҭ�ˡ��в����ձ���©������������Ͳ��ʯ�������ƾ��ơ����ܡ��Թܵȡ�
NaCl��Һ������ˮ�����ӡ�95���ƾ����������Լ���Ũ���ᡢ��Ҭ�ˡ��в����ձ���©������������Ͳ��ʯ�������ƾ��ơ����ܡ��Թܵȡ�
F��ʵ�鲽�裺��ĥ���Ƚ������˵���Һ����Һϡ��6�������Ĵ����ó����� �����������ܽ���ӱ��Ӿ��ú�ȥ����Һ����ȡ��DNA��DNA������
���Ķ����ϲ��ϻش��������⣺
��1����ĥʱ��ȡ10g��Ҭ�ˣ���������ʯӢɰ��____________ ��____________��
��2������Һϡ��6������Ŀ����______________________________________________��
��3��ȡ���������2mL 1mol��![]() NaCl ��Һ�У�ʹDNA�˵����ٴ��ܽ⣬�ټ�2mL���ӳ����ֹ������ֲ�������ϲ�ı��ӡ��ò����Ŀ���dz�ȥ__________��
NaCl ��Һ�У�ʹDNA�˵����ٴ��ܽ⣬�ټ�2mL���ӳ����ֹ������ֲ�������ϲ�ı��ӡ��ò����Ŀ���dz�ȥ__________��
��4����ν�ʣ����Һ�е�DNA��ȡ����? _____________________________________
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
_____________________________________________________��
��5�����֤����ȡ��ȷʵ��DNA����?________________________________________
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�071
�������й�DNA����ȡʵ����Ķ����ϣ�
A�����Ἣ���ȶ����ڽ�Ϊ���ҵĻ�ѧ���������غ�ø�������º������⡣���Ʊ�DNAʱҪ����DNAø��ˮ��DNA��ø�������Ƽ��������ƣ��Գ�ȥMg����ֹDNAø�ļ��
B�������е�DNA��RNA���������ھ��Ժ˵��ף��ɺ���͵�������ɣ�����ʽ���ڣ�DNA�˵�����1mol![]() NaCl��Һ���ܽ�Ⱥܴ���0.14mol��
NaCl��Һ���ܽ�Ⱥܴ���0.14mol��![]() NaCl��Һ�е��ܽ�Ⱥܵͣ���RNA�˵�������0.14mol��
NaCl��Һ�е��ܽ�Ⱥܵͣ���RNA�˵�������0.14mol��![]() NaCl��Һ��
NaCl��Һ��
C�������Ӵ�������ʹ�����ʱ��ԣ������ڱ��Ӳ��ڣ���DNA��Һ�м���2.5�������Ũ��Ϊ95���ľƾ����ɽ�DNA�����������ʱDNAʮ��ճ�������ò���������ȡ����
D��DNA��ǿ�ỷ���£�ˮ������������ǵ�С�������ʣ����������������Һ��Ӧ����������ɫ�����
E��ʵ���������е������������Һ��ʯӢɰ��0.14mol��![]() NaCl��Һ��1mol��
NaCl��Һ��1mol��![]() NaCl��Һ������ˮ�����ӡ�95���ƾ����������Լ���Ũ���ᡢ��Ҭ�ˡ��в����ձ���©������������Ͳ��ʯ�������ƾ��ơ����ܡ��Թܵȡ�
NaCl��Һ������ˮ�����ӡ�95���ƾ����������Լ���Ũ���ᡢ��Ҭ�ˡ��в����ձ���©������������Ͳ��ʯ�������ƾ��ơ����ܡ��Թܵȡ�
F��ʵ�鲽�裺��ĥ���Ƚ������˵���Һ����Һϡ��6�������Ĵ����ó����� �����������ܽ���ӱ��Ӿ��ú�ȥ����Һ����ȡ��DNA��DNA������
���Ķ����ϲ��ϻش��������⣺
��1����ĥʱ��ȡ10g��Ҭ�ˣ���������ʯӢɰ��____________ ��____________��
��2������Һϡ��6������Ŀ����______________________________________________��
��3��ȡ���������2mL 1mol��![]() NaCl ��Һ�У�ʹDNA�˵����ٴ��ܽ⣬�ټ�2mL���ӳ����ֹ������ֲ�������ϲ�ı��ӡ��ò����Ŀ���dz�ȥ__________��
NaCl ��Һ�У�ʹDNA�˵����ٴ��ܽ⣬�ټ�2mL���ӳ����ֹ������ֲ�������ϲ�ı��ӡ��ò����Ŀ���dz�ȥ__________��
��4����ν�ʣ����Һ�е�DNA��ȡ����? _____________________________________
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
_____________________________________________________��
��5�����֤����ȡ��ȷʵ��DNA����?________________________________________
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2012-2013ѧ���㽭ʡ����У��������һ���������������Ծ� ���ͣ��ۺ���
�Ķ����²��ϣ��ش�������⡣
��ѧ������˹���ֲ�������أ�IAA���ٽ������ġ���������˵��������ͼ��ʾ�����������������ϣ�����ϸ����ͨ���ź�ת���ٽ����ӱû����H+�ŵ�ϸ���ڣ�ϸ����pH�½������������ƻ�������ɳ���������ά�ص���˿�ɿ���ϸ������ѹ�½���ϸ����ˮ��ϸ��������ӳ�������������ת���������ش��������⣺
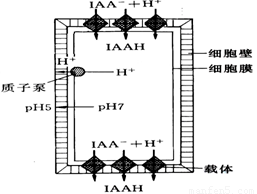
��1��������ֲ�D�ػ��Ƕ��D�أ�������ϸ������Ҫ������____ ��
��2����ͼ��֪�������ص������ص���_ ��
��3����ͼ���Ʋ��ϸ���Ͼ��е����뼤�ط��ӽ�ϵ����嵰�ܿ��ܾ��� ���ɲ�����Ϣ��ͼʾ��֪��ϸ�����������صķ�ʽ��__ ��
��4������ѧ������˹���ֲ�������أ�IAA���ٽ������ġ�����ѧ˵��������������Ĥ�ϵļ������嵰��Ϻ���ϸ���ڵ���ʹ��������Ϣת����ϸ�����ڣ�ʹ��������״̬�Ļ����������____________ _________ _____________��������ѧ֪ʶ���Ƹ����ۣ���
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010���Ĵ������Ͻ��츮��ѧ���������������� ���ͣ��ۺ���
��12�֣���ͼΪ�˵���Ⱦɫ���ͼ��X��YȾɫ����һ������ͬԴ��(��ͼ��IƬ��)���ò��ֻ���Ϊ��λ����һ�����Ƿ�ͬԴ��(��ͼ�еĢ�1����2Ƭ��)���ò��ֻ���Ϊ��λ����ش�

(1)�����Ѫ�Ѳ�����λ�ڼ�ͼ�е�____________Ƭ�Ρ�
(2)�ڼ��������γ����ӹ����У�x��YȾɫ����ͨ��������������������Ǽ�ͼ�е�____________Ƭ�Ρ�
(3)ij�ֲ����Ŵ�ϵ������ͼ������Ƹò��Ļ���ܿ���λ�ڼ�ͼ�е�____________Ƭ�Ρ�
(4)�������ij�������״�Ļ���A(a)λ�ڼ�ͼ��ʾX��YȾɫ���IƬ�Σ���ô�����״�ں����Ů�����б����͵ı���һ����ͬ��?�Ծ�һ����
__________ _
_________________________ _________ ��
��5������ij�������������Ի���ͬ����ʱ���ܺϳɣ�����G��g��λ�ڢ�Ƭ���ϣ���һ�Ե�λ����E��e��λ��һ�Գ�Ⱦɫ���ϡ��������ܺϳɸ����ʵ��ױ��ӽ�����һ�����ܺϳɸ����ʣ��Ӷ������ܺϳɸ������벻�ܺϳɸ����ʵı���Ϊ9��7���������ױ��Ļ�����Ϊ ��
 ��6����������Ⱥ�У����������ܴ�������ΪAA�ĸ���ռ24����Aa�ĸ���ռ72����aa�ĸ���ռ4���������ֻ����͵ĸ�����ijһ�����е�����������������ΪAA=Aa>aa�������Ժ�ij�����Ȼѡ������У�A��a����Ƶ�ʱ仯���ƿ��ܻ����������ڸ�����������������ͼ��ʾ��
��6����������Ⱥ�У����������ܴ�������ΪAA�ĸ���ռ24����Aa�ĸ���ռ72����aa�ĸ���ռ4���������ֻ����͵ĸ�����ijһ�����е�����������������ΪAA=Aa>aa�������Ժ�ij�����Ȼѡ������У�A��a����Ƶ�ʱ仯���ƿ��ܻ����������ڸ�����������������ͼ��ʾ��

 II(14��) ���Ķ����²��ϣ�
II(14��) ���Ķ����²��ϣ�
2008��5��12�գ��ҹ��Ĵ�ʡ�봨�ط���8��0�����𣬵����������ҹ�����è����ϡҰ���������Ҫ��Ϣ�غ���Ȼ�������ֲ���Ϊ���е�����������49����Ȼ�����������ܴ���è��������������������Ϣ�����������һЩ������Ϊ�еĶ�����è���������ǡ��ƺ����ȡ����ڵ���Ӱ�죬�����2 850��Ķ�Ĵ���è��Ϣ���ܵ������ƻ�������ش��������⣺
(1)�����������̬ϵͳ��˻������ƻ������������Իָ�����һ��ʵ˵���κ���̬ϵͳ�� �������ġ�
(2)������Ԯ�����У�һЩ�ٱ���Ⱦ�˽괯���괯������������Ƥ������Ľ��������һ�����Դ�Ⱦ��Ƥ�������������˵Ĺ�ϵ�� ��
(3) ������ͼ���ü�ͷ�����ֲ������������������è��Ⱥ�������

 ��4����ͼ��ʾ��̬ϵͳ�е�3������Ķ�����̼�ͷ������������������ͼ��a���ߴ����������������
����̬ϵͳ�ɷ֣���c���ߴ����������������
����̬ϵͳ�ɷ֣�������ͼ�����ܴ�������̬ϵͳ�ṹ�⣬��������̬ϵͳ�ɷֻ���
��
��4����ͼ��ʾ��̬ϵͳ�е�3������Ķ�����̼�ͷ������������������ͼ��a���ߴ����������������
����̬ϵͳ�ɷ֣���c���ߴ����������������
����̬ϵͳ�ɷ֣�������ͼ�����ܴ�������̬ϵͳ�ṹ�⣬��������̬ϵͳ�ɷֻ���
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com