
��12�֣�������������Ϣ�ⲡ��һ�ֵ������Ŵ��Լ�������Ҫ����Ϊ����������������Ϣ�⣨����������Щ�����粻���ƣ����� 100%�ᷢ�����䣬��Ϊ�᳦ֱ��������������� APC�����з�����һ�������ͻ�䣬�Ӷ�ʹ�䷭��IJ����е� 147λ�ľ������滻Ϊ�Ȱ���������֪������Ȱ������������ӷֱ�Ϊ CGC��CGU��CGA��CGG�� CAA��CAG��������ش��������⣺
��1����������APC�����з���ͻ��ļ������ ��
��2����ѧ���ڶԼ�����������Ϣ�ⲡ���²�������м��ʱ��Ӧʹ�� ��Ϊ̽�룬Ӧ�� ����������������ļ�����С�
��3����ͼΪij������������Ϣ�ⲡ���Ŵ�ϵ��ͼ����ػ����� A��a��ʾ��
�ٸò����²�����λ�� Ⱦɫ���ϵ� ���Ŵ�����
������4�����뻼��Ů�Ը�����䣬����һ�����к��ĸ����� �� 2�֣���
��ͼ�Т� 8�͢� 9�������ĸ����� ��
������ò���ij�����ķ�������1/10000����õ����ò����²��������Ƶ���� ��
��1��G//C
��2����ǵ��²������DNA���� DNA�����ӽ�
��3���ٳ� �� ��1/6 ��1/36 ��1/100
�������������
��1�����ڷ����˵����ͻ�䣬�ȽϾ�����Ȱ���������ӿ�����֪���������������ΪCGA��CGG��ͻ����������ΪCAA��CAG����֮����������G�滻����A�����Ӧ����Ƭ���У�����ͻ��ļ����ΪG//C��
��2����ѧ���ڶԼ�����������Ϣ�ⲡ���²�������м��ʱ��Ӧʹ�÷�����Ԫ�ر�ǵ��²������DNA������̽�룬������DNA�ӽ���
��3���ٷ���ϵ��ͼ��1�ź�2�������������ǵ�Ů��3�Ż�������ò�һ��Ϊ��Ⱦɫ�������Ŵ�����
����ǰһ�ʿ�����֪����3������Ϊaa����1�͢�2������ΪAa�����4������Ϊ1/3AA��2/3Aa���뻼��Ů�ԣ�aa����飬����һ�������к��ĸ���Ϊ2/3��1/2��1/2=1/6��
�ۢ�5�Ļ�����Ϊ1/3AA��2/3Aa����6��ĸ���������6������ΪAa�����5�͢�6��飬�������ĸ���Ϊ2/3��1/4=1/6����ˢ�8�͢�9�������ĸ���Ϊ1/6��1/6=1/36��
�����ڸò��ڸõ����ķ�����Ϊ1/10000�����²�����a���Ļ���Ƶ��Ϊ1/100��
���㣺���⿼�����ͻ�䡢�����Ŵ������й�֪ʶ�����ڿ��鿼��ʶͼ�������������֡�ͼ���Լ���ѧ��ʽ�ȶ��ֱ�����ʽȷ����������ѧ��������ݵ���������������ѧ֪ʶ��۵㣬ͨ���Ƚϡ��������ۺϵȷ�����ijЩ����ѧ������н��͡������������������жϻ�ó���ȷ�Ľ��ۣ�������ϵʵ�ʣ��ۺ�������ѧ֪ʶ�����Ȼ�����������е�һЩ����ѧ���⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
����ͼ��ʾ���������Ŵ���Ϣ�Ĵ��ݹ��̡����ͼ�ش��������⡣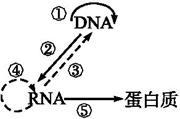
(1)�ٹ�����Ҫ��ԭ������������,ģ�����״�DNA����������������
(2)�ڹ��̱�ʾ��������,��Ҫ�������������н��еġ�
(3)��ת¼ø����������(�����)����,�ܽ��иù��̵�������Ŵ�����������____��������Ҫ����ø�Ĺ��̰���ͼ�е�����(�����)���̡�
(4)���ϸ�����Ŵ���Ϣ���������Ϊͼ�е���������(�����)����,����������������
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��12�֣�ÿ��2�֣�ij��ֲ��Ļ�ɫ�а�ɫ����ɫ����ɫ����A��a��B��b���Ե�λ�������Ŵ������ƣ������������Ի�ɫ��״���Ƶ����ּ�˵������ͼ��ʾ��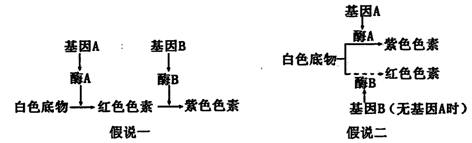
��ش�
��1����˵һ���������� ����ʱ����ɫ����Ϊ��ɫ����˵������������__________����ʱ����ɫ����Ϊ��ɫ��
��2����ѡȡ������ΪAaBb���ϻ�ֲ��Ϊ�ױ������Խ���
������˵һ������F1��ɫ����״������Ϊ ��F1�а��Ļ������� �֡�
������˵��������F1��ɫ����״������Ϊ ��F1�к컨�Ļ�����Ϊ ����ȡF1�컨ֲ������Խ�����F2�г��ֺ컨�ı���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��14�֣�����ָ���ⴥ��������棬�������ⷴ�䡣�䷴�仡ʾ��ͼ���¡�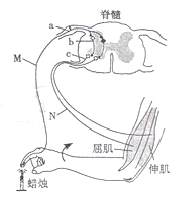
��1��ͼ����Ԫa�������˷ܴ�����ĩ��ʱ������ͻ��ǰĤ�� �ͷ����ʣ��õ���ʹ��Ԫb�˷ܡ���Ԫb���嶯��һ��������Ԫc�˷ܣ����յ�������������
��2�����������ߵ���ϸ���ͼ���ϸ����������������������˶����ܣ���ԭ��������� �����쳣������ġ�
��3��ͼ��M���˷�ʱ���˴�����άĤ����ĵ�λ����Ϊ ����N���ܴ̼������˷ܣ�������Ԫb��
����С����ޡ���Ĥ��λ�ı仯����ԭ���� ��
��4����ָ���ⴥ����������ֲ�Ƥ�����ף�����ΪƤ��ëϸѪ�����ź�ͨ�����ӣ�
������֯��϶Һ����ۡ�����ָ�˿ڸ�Ⱦ����������Һ������ϸ����ɱ�����ʵ��������ֺ����˹������� ���ߡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
(10��)(2012����̨���)���±���ʾΪij�������������䷽����ش�
| �ɷ� | ���� |
| NaNO3 | 3 g |
| K2HPO4 | 1 g |
| KCl | 0.5 g |
| MgSO4��7H2O | 0.5 g |
| FeSO4 | 0.01 g |
| (CH2O) | 30 g |
| H2O | 1 000 mL |
| ��ù�� | 0.1��λ |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��8�֣���ͼ�ס��ҷֱ��ʾij�������ΪAaBbdd�Ĵ��Ըߵȶ���ϸ�����ѹ�����ijʱ�ڵ�Ⱦɫ�����ʾ��ͼ�������γ�ʱϸ����Ⱦɫ�������仯����ͼ��
���ͼ�ش���������⡣
(l)ͼ����ʾϸ������Ϊ____����ϸ���к�ͬԴȾɫ��͵�λ����ֱ�Ϊ___�Ժ�____�ԡ���l��Ⱦɫ���ʾXȾɫ�壬��2�ź�4��Ⱦɫ��ֱ����____��
(2)�ϵ¶����Ŵ�������ͨ��������һ�η��Ѻ��ڵ�____��ʵ�ֵġ�
(3)ͼ���еĺ�����������У�____��ʾ����b��b�ķֿ�ʱ�ڡ�
(4)������ͼ�ķ������Ӧ�ķǵ�λ�������ͬԴȾɫ��������ϵ�ϸ��ʱ��ͼ��������������ʾ��ͼ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��12�֣���ش��ϵ¶��Ŵ����ɼ�����������������Ӧ�õ�������⣺
���㶹�ǽ����Ŵ�ʵ���������ϣ����û�ɫԲ���㶹����ɫԲ���㶹�����ӽ�ʵ��[��ɫ��Y������ɫ��y��Ϊ���ԣ�Բ����R����������r��Ϊ����]�������Ӵ������Ͱ�ÿ�������״����ͳ�ƣ��������ͼ��ʾ����ش����⣺
|

�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��8�֣���ѧ�ҽ��˵��������ػ�����˾���DNA���ӽ������飬���ɹ����ڴ˾��е��Ա�����ڽ��л��̵IJ��������У���ʹ���ض�������ø�и�Ŀ�Ļ�������������������ɸѡ����֪����ø���ʶ�����к��е��ǡ�G��GATCC�� ������ø���ʶ�����к��е��ǡ���GATC������ͼ�ش�
�Ź��̢ٱ�ʾ���Dz�ȡ �ķ�������ȡĿ�Ļ���
�Ƹ���ͼʾ�������ڹ������������������У�Ӧ������ø �и�������������ø ø�и�Ŀ�Ļ���������ø�и�Ŀ�Ļ������������γɵ����ĩ��ͨ�� ԭ��������ӡ�
���˵Ļ���֮��������˾���DNA���ӽ������飬ԭ���� ��
��������������ػ�������ϸ�����ڳɹ���������Ϊ ��д��Ŀ�Ļ�����ϸ���б���Ĺ��� ��
�ɽ��õ��Ĵ˾�BͿ����һ�����а�����ù�ص��������ϣ��ܹ������ģ�˵���ѵ����� ����֮��û�е��롣
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ͼ�DZ�ʾij�������ϸ����Ⱦɫ�弰DNA������仯������ͼ�����ݴ�����ͼ�ش��������⣺��ע������������������ϸ�����ѵĸ���ʱ�ڣ�����Ĵ�С����ʱ�������ʱ�䲻�ɱ�����
��ͼ�д���Ⱦɫ����������仯�������� ��
�ƽ���ͼ��8����ʱ��ϸ��֮������Ϣ�����������Ϣ������ϸ���ṹ�� ����صijɷ���
��3��ͼ�д�8--13��ʱ�ڱ�ʾϸ���� ���̡�ϸ���ں���ͬԴȾɫ��������� ��
������������ϸ����Ⱦɫ����Ϊ20������һ��ϸ�����е�DNA��������9��12ʱ��Ϊ ����
����˿����ѷ����ں��������ֵ� �����С�
��8��������������� ��
��7��Ⱦɫ�塢Ⱦɫ���塢DNA�ı���Ϊ1��2��2��������
��8��7-8����ϸ����Ⱦɫ����DNA������1-4����ϸ����Ⱦɫ����DNA������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com