
¾ŻĶ¼»Ų“šĪŹĢā”£
(1)²ĪÓėĶ¼¼×¹ż³ĢµÄĆøÖ÷ŅŖŹĒ________”£
(2)Ķ¼ŅŅŹĒĶ¼¼×ÖŠ¢ŁŗĶ¢Ś·Ö×Ó֊ijŅ»µ„Ī»µÄ·Å“óĶ¼£¬ŌŚ¢ŁŗĶ¢Ś·Ö×ÓÖŠ£¬B²æ·ÖµÄĒų±šÖ÷ŅŖŹĒ_______________________________________________________________£»
Ķ¼¼×ÖŠ¢ŁŌŚŌŗĖĻø°ūÖŠÖ÷ŅŖ“ęŌŚÓŚ________ÄŚ£»¢ŚŌŚÕęŗĖĻø°ūÖŠÖ÷ŅŖ“ęŌŚÓŚ________ÖŠ”£
(3)Ķ¼±ū¹ż³Ģ·¢ÉśµÄ³”ĖłŹĒ________£¬ŗĻ³É¢ŪµÄ³”ĖłÖ÷ŅŖŹĒ________”£
(4)Ķ¼¶”ÖŠ¢ÜµÄĆū³ĘĪŖ________£»“ÓĶ¼ÖŠæÉŅŌ擳ö£¬tRNAÓė°±»łĖį½įŗĻµÄ¹ż³ĢÖŠÓŠ________Éś³É”£
(5)Ķ¼±ūÖŠ¢Ś·Ö×Ó±Č¢Ū·Ö×Ó________(Ģī”°“ó”±»ņ”°Š””±)µĆ¶ą£»Ķ¼Ź¾tRNAÖŠ£¬GŗĶCµÄŹżÄæŹĒ·ńĻąµČ£æ________”£
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
Ķ¼1±ķŹ¾±±Ī³50”ćijµŲĒų²»Ķ¬ŌĀ·ŻÄø¼¦Ę½¾ł²śµ°ŹżµÄ²Ø¶ÆĒéæö£¬Ķ¼2ŹĒÄø¼¦·±Ö³»ī¶ÆµÄÉśĄķµ÷½Ś¹ż³ĢŹ¾ŅāĶ¼(¼×”¢ŅŅ”¢±ū±ķŹ¾Ę÷¹Ł£¬a”¢b”¢c±ķŹ¾ĻąÓ¦¼¤ĖŲ)”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
Ķ¼1”””” ”” Ķ¼2
(1)ÓÉĶ¼1æÉÖŖ£¬Ó°ĻģÄø¼¦ŌĀ²śµ°ŹżµÄÖ÷ŅŖ»·¾³Ģõ¼žŹĒ________”£
(2)Ķ¼2ÖŠ¼¤ĖŲb”¢Ę÷¹Ł±ūµÄĆū³Ę·Ö±šŹĒ________”¢________”£
(3)Ķ¼2ÖŠc”ś¼×”¢c”śŅŅµÄµ÷½Ś·½Ź½³ĘĪŖ_______”£Äø¼¦·±Ö³»ī¶ÆµÄÉśĄķµ÷½ŚŹōÓŚ µ÷½Ś”£
(4)Ķ黳±½·ÓŹĒŅ»ĄąĻ“µÓ¼Į£¬Ęä·Ö×Ó½į¹¹ĪČ¶Ø£¬¾ßÓŠĄąĖĘ¼¤ĖŲcµÄÉśĄķŠ§Ó¦”£³¤ĘŚĖĒĪ¹±»Ķ黳±½·ÓĪŪČ¾µÄĖĒĮĻ£¬»į¶ŌĘ÷¹Ł¼×”¢ŅŅµÄ·ÖĆŚ»ī¶Æ²śÉś________×÷ÓĆ£¬“Ó¶ųŅżĘš¼¤ĖŲc·ÖĆŚĮæ________,Ę÷¹Ł±ū½«»į³öĻÖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
ĻĀĮŠĪŖČĖĢå¼øÖÖĆāŅßĻø°ū²ĪÓė»śĢåĆāŅßµÄĮ½ÖÖ»śÖʵÄĶ¼½ā£¬ĘäÖŠµÄ×ÖÄø±ąŗűķŹ¾Ļø°ūĄąŠĶ»ņÓÉĖüĆĒ²śÉśµÄijŠ©ĪļÖŹ£¬Źż×Ö±ķŹ¾×÷ÓĆ¹ż³Ģ”£Ēė¾ŻĶ¼¼°ĖłŃ§ÖŖŹ¶»Ų“šĪŹĢā£ŗ
 1£®¹ż³Ģ¢Ł ¢Ś ¢Ū±ķŹ¾ÓÉ_______ĮÜ°ĶĻø°ū·¢»ÓµÄĆāŅß×÷ÓĆ£¬³ĘĪŖ________ĆāŅߣ»¹ż³Ģ¢ÜÖĮ¢ąŌņ±ķŹ¾ÓÉ_______ĮÜ°ĶĻø°ū·¢»ÓµÄĆāŅß×÷ÓĆ£¬³ĘĪŖ____________ĆāŅߣ¬Į½ÖÖĆāŅß·½Ź½ĶłĶłŹĒ×ŪŗĻ“ęŌŚµÄ”£
1£®¹ż³Ģ¢Ł ¢Ś ¢Ū±ķŹ¾ÓÉ_______ĮÜ°ĶĻø°ū·¢»ÓµÄĆāŅß×÷ÓĆ£¬³ĘĪŖ________ĆāŅߣ»¹ż³Ģ¢ÜÖĮ¢ąŌņ±ķŹ¾ÓÉ_______ĮÜ°ĶĻø°ū·¢»ÓµÄĆāŅß×÷ÓĆ£¬³ĘĪŖ____________ĆāŅߣ¬Į½ÖÖĆāŅß·½Ź½ĶłĶłŹĒ×ŪŗĻ“ęŌŚµÄ”£
2£®Ķ¼ÖŠ±ąŗÅAŹĒ Ļø°ū£¬±ąŗÅDŹĒ__________Ļø°ū£»EŹĒ ”£±ąŗÅBĻø°ūµÄ×÷ÓĆŹĒ ”£
3£®·ĒĢŲŅģŠŌĆāŅßÖ÷ŅŖĶعż°ūĶĢŠĪ³ÉĶĢŹÉÅŻ£¬Ļø°ūÄŚµÄijŅ»½į¹¹Ėę¼“»įÓėĶĢŹÉÅŻČŚŗĻ£¬ŌņøĆ½į¹¹Ćū³ĘŹĒ £¬ĘäÄŚ²æɱƚ²”ŌĢåµÄĪļÖŹŹĒ ”£
4£®¾ŻĻĀĶ¼·ÖĪö£¬ŌŚµŚ1ğČ£¬Ōģ³ÉHIVµÄÅضČĆ÷ĻŌĻĀ½µµÄŌŅņŹĒ TĻø°ū¶ŌHIVµÖæ¹×÷ÓƵĻśĄķŹĒ ”£µŚ1Äźµ½µŚ9ğČ£¬HIVµÄÅØ¶Č±ä»ÆŹĒ_________£¬ŌŅņŹĒ___________________________________”£Čō²»¼°Ź±ÖĪĮĘ£¬°¬×Ģ²”»¼Õß²»Äܳ¤Ź±¼äÉś“ę£¬ŅņĪŖĘä_________________»ł±¾É„Ź§”£TĮÜ°ĶĻø°ūµÄ¶ąÉŁŗĶHIVÅضČÖ®¼ä³Ź_________¹ŲĻµ”£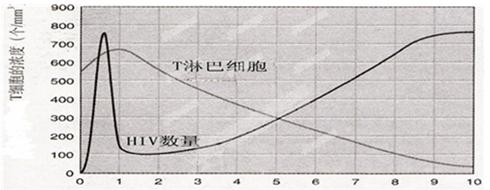
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
ĻĀĶ¼±ķŹ¾ĻĀĒšÄŌÉń¾Ļø°ū”¢“¹ĢåĻø°ū”¢¼×דĻŁĻø°ū¼°ĖüĆĒ·ÖĆŚµÄ¼¤ĖŲÖ®¼äµÄ¹ŲĻµ”£Ēė»Ų“šĻĀĮŠĪŹĢā”£
1.Ķ¼ÖŠµÄĻĀĒšÄŌÉń¾Ļø°ū³ż¾ßÓŠÉń¾Ļø°ūµÄ¹¦ÄÜĶā£¬»¹¾ßÓŠ ¹¦ÄÜ”£
2.ČĖŌŚŗ®ĄäµÄ»·¾³ÖŠ£¬ĪļÖŹ¼×¼“ µÄŗ¬Įæ»įŌö¼Ó£¬Ó°ĻģĖüĖł×÷ÓƵİŠĻø°ūµÄ»ī¶Æ£¬×īÖÕŌģ³ÉĪļÖŹ±ūµÄŗ¬Įæ £¬¼Ģ¶ųÓÖŅżĘš¼×ŗĶŅŅµÄ·ÖĆŚĮæ £¬ÕāÖÖµ÷½Ś·½Ź½³ĘĪŖ µ÷½Ś”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
»ĘÉ«Ō²Į£Ķć¶¹ÓėĀĢÉ«Ō²Į£Ķć¶¹½ųŠŠŌÓ½»£¬¶ŌĘä×Ó“ś±ķĻÖŠĶ°“Ćæ¶ŌĻą¶ŌŠŌד½ųŠŠ·ÖĪöŗĶĶ³¼Ę£¬½į¹ūČēĻĀĶ¼ĖłŹ¾£ŗ£Ø»ĘÉ«”¢ĀĢÉ«ÓĆY”¢y±ķŹ¾£¬Ō²Į£”¢ÖåĮ£ÓĆR”¢r±ķŹ¾£©
£Ø1£©Ē×±¾µÄ»łŅņŠĶ£ŗ»ĘÉ«Ō²Į£Ķć¶¹ŹĒ £¬ĀĢÉ«Ō²Į£Ķć¶¹ŹĒ ”£
£Ø2£©ŌÓ½»ŗó“śÖŠÄÜĪȶØŅÅ“«µÄŹżĮæÕ¼×ÜŹżµÄ ”£
£Ø3£©ŌÓ½»ŗó“śÖŠ£¬ÖŲ×饹ŠĶĖłÕ¼±ČĄżŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
ĻĀĶ¼ĖłŹ¾ŌŚŅ»¶ØCO2ÅضČŗĶĪĀ¶ČĢõ¼žĻĀ£¬Ä³ŃōÉśÖ²ĪļŗĶŅõÉśÖ²Īļ¹āĒæ¶ČŗĶ¹āŗĻ×÷ÓĆŗĻ³ÉĮæ£ØÓĆCO2ĪüŹÕĖŁĀŹ±ķŹ¾£©µÄ¹ŲĻµĶ¼”£Ēėøł¾ŻĶ¼»Ų“š£ŗ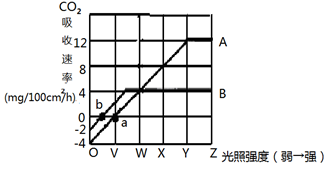
£Ø1£©ĒśĻßA±ķŹ¾µÄŹĒ £ØŅõÉś/ŃōÉś£©Ö²ĪļµÄŹÜ¹āĒæ¶ČŗĶ¹āŗĻ×÷ÓĆŗĻ³ÉĮæµÄ¹ŲĻµ”£
£Ø2£©a”¢bµć±ķŹ¾ ”£
£Ø3£©Ņ¶Ć껿ĪŖ25cm2µÄŃōÉśÖ²ĪļŅ¶Ę¬ŌŚ¹āĒæ¶ČĪŖY£¬¹āÕÕŹ±¼äĪŖ1Š”Ź±µÄĢõ¼žĻĀ£¬Ęä¹āŗĻ×÷ÓĆŗĻ³ÉĮæĪŖ mgĘĻĢŃĢĒ”££ØŠ”Źżµćŗó±£ĮōŅ»Ī»£©
£Ø4£©½«øĆŅõÉśÖ²ĪļŅ¶Ę¬ĻČŌŚ¹āÕÕĒæ¶ČĪŖXµÄĢõ¼žĻĀÕÕÉä Š”Ź±£¬Č»ŗó·ÅÓŚ°µ“¦12Š”Ź±£Ø¹āÕÕĒæ¶ČĪŖ0£©£¬Äܹ»Ź¹Ņ¶µÄøÉĪļÖŹĮæÓė¹āÕÕĒ°ĻąĶ¬”£
£Ø5£©µ±¹āÕÕĒæ¶ČĪŖYŹ±£¬ĻŽÖĘAÖ²Īļ¹āŗĻ×÷ÓƵÄÖ÷ŅŖ»·¾³ŅņĖŲĪŖ____________£¬“ĖŹ±Ņ¶ĀĢĢåÖŠADPµÄŌĖ¶Æ·½ĻņŹĒ“Ó µ½ ”£
£Ø6£©ŌŚ¹āŗĻ×÷ÓĆÖŠ£¬¹ā·“Ó¦½×¶ĪÄÜĮæµÄ×Ŗ±ä¹ż³ĢŹĒ £¬ŌŚÕāøö½×¶ĪµÄ²śĪļÖŠ£¬ÄÜĪŖ°µ·“Ó¦¹©ÄܵďĒ £ØĢīĪļÖŹ£©”£
£Ø7£©ĒėŠ“³öĻø°ūÓŠŃõŗōĪüµÄ×Ü·“Ó¦Ź½_______________________________”£
£Ø8£©Čē¹ū½«Ņ»ÖźĀĢÉ«Ö²ĪļŌŌÅąŌŚŗ¬H218OµÄĶźČ«ÅąŃųŅŗÖŠ£¬øųÓč³ä×ćµÄ¹āÕÕ£¬¶ĢŹ±¼äÄŚĻĀĮŠĪļÖŹÖŠÄÜŗ¬18OµÄÓŠ______________Ļī”£
¢ŁÖÜĪ§æÕĘųµÄŃõĘų ¢ŚÖÜĪ§æÕĘųµÄ¶žŃõ»ÆĢ¼
¢ŪÖÜĪ§æÕĘųµÄĖ®·Ö×Ó ¢Ü¹āŗĻ×÷ÓĆÉś³ÉµÄĘĻĢŃĢĒ
| A£®Ņ»Ļī | B£®¶žĻī | C£®ČżĻī | D£®ĖÄĻī |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
ĻĀĶ¼±ķŹ¾Ä³Ņ»¹Ū»ØÖ²Īļ»ØÉ«ŠĪ³ÉµÄŅÅ“«»śĄķ£¬ĘäÖŠ×ÖÄø±ķŹ¾æŲÖʶŌÓ¦¹ż³ĢĖłŠčµÄ»łŅņ£¬ĒŅø÷µČĪ»»łŅņ±ķĻÖ³öĶźČ«ĻŌŠŌ£¬·ĒµČĪ»»łŅņ¼ä¶ĄĮ¢ŅÅ“«”£Čō×ĻÉ«É«ĖŲÓėŗģÉ«É«ĖŲĶ¬Ź±“ęŌŚŹ±£¬Ōņ±ķĻÖĪŖ×ĻŗģÉ«”£Ēė»Ų“š£ŗ
£Ø1£©øĆÖ²Īļ»ØÉ«µÄŅÅ“«×ńŃ ¶ØĀÉ”£
£Ø2£©ČōĶ¬Ź±æ¼ĀĒČż¶ŌµČĪ»»łŅņ£¬ŌņÄܲśÉśŗ¬ŗģÉ«É«ĖŲµÄÖ²Öź»łŅņŠĶÓŠ ÖÖ£¬×Ļ»ØµÄÖ²Öź»łŅņŠĶŹĒ ”£
£Ø3£©ĻÖÓŠ“æŗĻ×Ļ»ØµÄÖ²ÖźÓė“æŗĻŗģ»ØµÄÖ²ÖźŌÓ½»£¬ĖłµĆF1µÄ±ķĻÖŠĶĪŖ ”£
F1×Ō½»£¬ŌņF2ÖŠ°×»ØµÄÖ²ÖźĖłÕ¼µÄ±ČĄżŹĒ £¬×Ļŗģ»ØµÄÖ²ÖźĖłÕ¼±ČĄżŹĒ ”£
£Ø4£©ŅŃÖŖøĆÖ²ĪļĪŖ¶ž±¶Ģå£¬Ä³Ö²ÖźÄ³ŠŌד³öĻÖĮĖæÉŅÅ“«µÄŠĀ±ķĻÖŠĶ£¬ĒėÉč¼ĘŅ»øö¼ņµ„ŹµŃ饓¼ų¶ØÕāøöŠĀ±ķĻÖŠĶµÄ³öĻÖŹĒÓÉÓŚ»łŅņĶ»±ä»¹ŹĒČ¾É«Ģå×é¼Ó±¶ĖłÖĀ£æ£ØŠ“³öŹµŃéĖ¼Ā·£© ”£
£Ø5£©Ä³»ØÅ©Ö»ÓŠ“æŗĻ×Ļ»ØµÄÖ²ÖźŗĶ“æŗĻŗģ»ØµÄÖ²Öź£¬Ļ£ĶūÄÜŌŚ×ī¶ĢŹ±¼äÄŚÅąŃų³öæÉĪȶØŅÅ“«µÄ°×»ØÖ²Öź£¬æɲÉÓƵÄÓżÖÖ·½·ØŹĒ £¬øĆÓżÖÖ·½·ØŅĄ¾ŻµÄÖ÷ŅŖŌĄķŹĒ ”£
£Ø6£©øĆÖ²ĪļµÄ»ØŅ׏Üŗ¦³ęæŠŹ³£¬“Ó¶ųÓ°Ļģ»ØµÄĘ·ÖŹ£¬ĪŖĮĖøÄĮ¼øĆÖ²ĪļµÄæ¹³ęŠŌ£¬Ķس£“ÓĘäĖūĪļÖÖ»ńµĆ »łŅņ£¬½«ĘäÓėŌĖŌŲĢå½įŗĻ£¬¹¹½Ø £¬Č»ŗóµ¼ČėøĆÖ²ĪļµÄĢåĻø°ūÖŠ£¬¾ ¼¼Źõ»ńµĆ“óĮæµÄ×Ŗ»łŅņæ¹³ęÖ²Öź”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
·ÖĪöÓŠ¹ŲŅÅ“«²”µÄ׏ĮĻ£¬»Ų“šĪŹĢā£ŗ
׏ĮĻ£ŗµ÷²éijÖÖŅÅ“«²”µĆµ½ČēĻĀĻµĘ×Ķ¼£¬¾·ÖĪöµĆÖŖ£¬Į½¶Ō¶ĄĮ¢ŅÅ“«ĒŅ±ķĻÖĶźČ«ĻŌŠŌµÄ»łŅņ£Ø·Ö±šÓĆ×ÖÄøAa”¢Bb±ķŹ¾£©ÓėøĆ²”ÓŠ¹Ų£¬ĒŅ¶¼æÉŅŌµ„¶ĄÖĀ²””£ŌŚµ÷²é¶ŌĻó֊ƻӊ·¢ĻÖ»łŅņĶ»±äŗĶČ¾É«Ģå±äŅģµÄøöĢ唣Ēė»Ų“šĻĀĮŠĪŹĢā:
¢ÅøĆÖÖŅÅ“«²”µÄŅÅ“«·½Ź½ŹĒ ”£
¢Ę¼ŁÉ赌¢ń“śøöĢåµÄĮ½¶Ō»łŅņÖŠ¾łÖĮÉŁÓŠŅ»¶Ō»łŅņŹĒ“æŗĻµÄ£¬¢ņ-3µÄ»łŅņŠĶĪŖAAbb£¬ĒŅ¢ņ-3Óė¢ņ-4µÄŗó“ś¾łÕż³££¬Ōņ¢ó-1µÄ»łŅņŠĶĪŖ ”£¢ņ-2µÄ»łŅņŠĶĪŖ ”£
¢ĒŌŚÉĻŹö¼ŁÉčµÄĒéæöĻĀ£¬Čē¹ū¢ņ-2Óė¢ņ-5»éÅ䣬Ęäŗó“śŠÆ“ųÖĀ²”»łŅņµÄøÅĀŹĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ×ŪŗĻĢā
£ØĆææÕ2·Ö£¬¹²12·Ö£©ĻĀ±ķŹĒH2O2ŌŚ²»Ķ¬Ģõ¼žĻĀµÄ·Ö½ā”£¾Ż±ķ»Ų“š£ŗ
| ŹŌ¹ÜŗÅ | “¦Ąķ | ĻÖĻó |
| 1 | 2 mL H2O2£«2µĪÕōĮóĖ® | ²śÉśĘųÅŻŗÜÉŁ |
| 2 | 2 mL H2O2£«2µĪFeCl3 | ²śÉśĘųÅŻ½Ļ¶ą |
| 3 | 2 mL H2O2£«2µĪøĪŌąŃŠÄ„Ņŗ | ²śÉśĘųÅŻŗܶą |
| 4 | 2 mL H2O2£«2µĪ(Öó·Š)øĪŌąŃŠÄ„Ņŗ | »ł±¾Ķ¬1 |
| 5 | 2 mL H2O2£«2µĪøĪŌąŃŠÄ„Ņŗ£«2µĪ5%HCl | »ł±¾Ķ¬1 |
| 6 | 2 mL H2O2£«2µĪøĪŌąŃŠÄ„Ņŗ£«2µĪ5%NaOH | »ł±¾Ķ¬1 |
| 7 | | »ł±¾Ķ¬3 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com