
ijѧ��ʵ��С����������µ�̽����ĸ��ϸ��������ʽ��ʵ�飬��������д���е�һЩ�հ�֮����
ʵ������ ̽����ĸ��ϸ�������ķ�ʽ
��һ�������뷽������ͼʾ����װ�ã�ÿ��һ��ʱ���¼ʵ������
��1����ʵ����Ա����� ��
��2��Aƿ������Լ���_________________��Cƿ��Eƿ������Լ���____ _____��
(��)��������
| װ�� ���� | �� | �� |
| CO2 | �� | �� |
| �ƾ� | �� | �� |
��1������
��2��NaOH ����ʯ��ˮ����������ݷ���ˮ��Һ��
(3����ƿ�е����������꣨2�֣�
���������������ͼ��ʵ����Ա�����������Aƿ������Լ���NaOH��Cƿ��Eƿ������Լ��dz���ʯ��ˮ��Dƿ��ں�Ҫ����һ��ʱ������ͨEƿ����ԭ���ǽ�ƿ�е����������ꡣ
���㣺̽����ĸ��ϸ��������ʽ��ʵ��
���������⿼����ѧ����ʵ������������Ѷ����У�����Ĺؼ�����ȷ��ĸ����ϸ�������ķ�ʽ��


 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��2012���Ϻ����ֶ���������������ģ�������Ծ����������� ���ͣ��ۺ���
��12�֣��ش�����������ѧʵ������⡣
��У�2n=16��Ϊ������ֲ�Ϊ�Ƚϲ�ͬ������������и���ϸ������ָ��������Ұ�ڷ�����ϸ����ռϸ�������İٷֱȣ���Ӱ�죬ij�о���ѧϰС����������ʵ�飬�������¡�
�ٽ���е��ϸ�ȥ������ˮ��������ȡ����
�ڽ���з���ͬʱת��Ũ��Ϊ0��01%��0��1%��ˮ������Һ�У��ֱ�����24h��36h��48h����ˮ���ش���ֹͣ����ת����ˮ�зֱ�����0h��12h��24h��36h��
�ۼ�ȡ���⣬�ÿ�ŵ�̶�Һ����3����ˮ�Ҵ���1�ݱ�������ȣ��̶�8h��Ȼ���������1mol/L������Һ��5~8min��
�ܽ�����ȡ��������ʢ����ˮ����������Ưϴ��
����ʯ̼��-Ʒ���Լ�Ⱦɫ��
����Ƭ�����죻���������ա�
��ͬ�����������ϸ������ָ����%�����±��������ش����⡣
| ��ˮ������ҺŨ�ȣ�%�� | ����ָ�� ��%�� ʱ�䣨h�� | ��ˮ����ʱ�䣨h�� | |||
| 0 | 12 | 24 | 36 | ||
| 0.01 | 24 | 10��71 | 13��68 | 14��19 | 14��46 |
| 36 | 9��94 | 11��99 | 13��59 | 13��62 | |
| 48 | 7��98 | 10��06 | 12��22 | 11��97 | |
| 0.1 | 24 | 7��74 | 9��09 | 11��07 | 10��86 |
| 36 | 6��12 | 7��87 | 9��98 | 9��81 | |
| 48 | 5��97 | 6��68 | 7��98 | 8��56 | |

�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2015��㶫ʡ��һ��ѧ����ĩ���������Ծ��������棩 ���ͣ��ۺ���
ijѧ��ʵ��С����������µ�̽����ĸ��ϸ��������ʽ��ʵ�飬��������д���е�һЩ�հ�֮����
ʵ������ ̽����ĸ��ϸ�������ķ�ʽ
��һ�������뷽������ͼʾ����װ�ã�ÿ��һ��ʱ���¼ʵ������
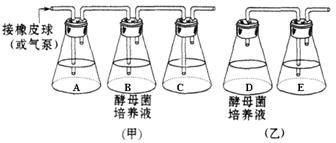
��1����ʵ����Ա����� ��
��2��Aƿ������Լ���_________________��Cƿ��Eƿ������Լ���____ _____��
(��)��������
|
װ�� ���� |
�� |
�� |
|
CO2 |
�� |
�� |
|
�ƾ� |
�� |
�� |
(��)����
��3��Dƿ��ں�Ҫ����һ��ʱ������ͨEƿ����ԭ���ǣ�____ ___________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2011-2012ѧ���Ϻ����ֶ���������������ģ�������Ծ��������棩 ���ͣ��ۺ���
��12�֣��ش�����������ѧʵ������⡣
��У�2n=16��Ϊ������ֲ�Ϊ�Ƚϲ�ͬ������������и���ϸ������ָ��������Ұ�ڷ�����ϸ����ռϸ�������İٷֱȣ���Ӱ�죬ij�о���ѧϰС����������ʵ�飬�������¡�
�ٽ���е��ϸ�ȥ������ˮ��������ȡ����
�ڽ���з���ͬʱת��Ũ��Ϊ0��01%��0��1%��ˮ������Һ�У��ֱ�����24h��36h��48h����ˮ���ش���ֹͣ����ת����ˮ�зֱ�����0h��12h��24h��36h��
�ۼ�ȡ���⣬�ÿ�ŵ�̶�Һ����3����ˮ�Ҵ���1�ݱ�������ȣ��̶�8h��Ȼ���������1mol/L������Һ��5~8min��
�ܽ�����ȡ��������ʢ����ˮ����������Ưϴ��
����ʯ̼��-Ʒ���Լ�Ⱦɫ��
����Ƭ�����죻���������ա�
��ͬ�����������ϸ������ָ����%�����±��������ش����⡣
|
��ˮ������ҺŨ�ȣ�%�� |
����ָ�� ��%�� ʱ�䣨h�� |
��ˮ����ʱ�䣨h�� |
|||
|
0 |
12 |
24 |
36 |
||
|
0.01 |
24 |
10��71 |
13��68 |
14��19 |
14��46 |
|
36 |
9��94 |
11��99 |
13��59 |
13��62 |
|
|
48 |
7��98 |
10��06 |
12��22 |
11��97 |
|
|
0.1 |
24 |
7��74 |
9��09 |
11��07 |
10��86 |
|
36 |
6��12 |
7��87 |
9��98 |
9��81 |
|
|
48 |
5��97 |
6��68 |
7��98 |
8��56 |
1��������С������������1mol/L������Һ�С��������� ��
2�������Ϊ�˻����Ӧ�Ĺ۲���Ұ������ʱ��ȷ�IJ��������� ��
3��������ѧ��֪ʶ�Ʋ⣬ʯ̼��-Ʒ���Լ���1�� ��Ⱦ�ϡ�
4��Ϊ��ͳ�����ݸ��ӿ�ѧ������ʱӦ��ȡ�ķ����� ��
5�������ϱ�������Եó����³������ۣ�
��Ũ��Ϊ ��ˮ������Һ�յ����ϸ������ָ���ϸߡ�
�ڱ�ʵ��ĸ��ִ����У����ϸ������ָ������ѷ�����
����ֻ���Ǵ��������е�1������������ Ϊ�Ա�������
�����£����ϸ������ָ����%��������ͼ��ʾ����2�֣�

6�����Ϸ���ѧ���ڲ���������������Ƭ������ϸ��Ⱦɫ��ӱ�����ܵ�ԭ����
(2��)��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
��09�ع��е��У���8�֣�ijѧ��ʵ��С����������µ�̽����ĸ��ϸ��������ʽ��ʵ�飬��������д���е�һЩ�հ�֮����
ʵ�����ƣ�̽����ĸ��ϸ�������ķ�ʽ
��һ�������뷽������ͼʾ����װ�ã�ÿ��һ��ʱ���¼ʵ������

��1��Aƿ������Լ���_________________����Ŀ����________________��
��2��Cƿ��Eƿ������Լ���_________����������__________________��
(��)��������
װ�� ���� | �� | �� |
CO2 | �� | �� |
�ƾ� | �� | �� |
(��)����
��3����μ������װ���оƾ��IJ��������____________________________________________________��
��4��Dƿ��ں�Ҫ����һ��ʱ������ͨEƿ����ԭ���ǣ�___________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com