
��ͼ��DNA����ijһ�������е��Ұ���ͱ��������ʾ��ͼ�������ش�
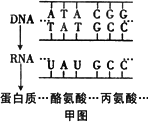
��1����ͼ�к���________�ֺ����________���Ƿ��ӣ����������������________����ͼ��ʾ��DNA���Ŵ���Ϣ��________��________���̡�
��2����ij����������3��������290����������ɵģ���ô������ҪDNA��________�����������Ƹõ����ʵĺϳɣ������Ҫ________�ְ����ᡣ
��3���Ҹβ�����һ��Լ��3200��������������ɵ�˫��DNA���������ֲ����ĸ��Ʒ�ʽ��Ϊ���⣬��Ҫ��������ͼ��ʾ����ͬ�ڼ�ͼ�����������ڣۡ�����________���̡�
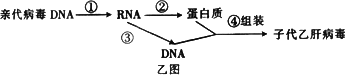
��4���������ķ����������ϸ���Ŵ���Ϣ�Ĵ��ݹ���________��
��5������DNAʱ��һ���Ƚ���������������ʵ���Ũ��Ϊ0.015 mol��L��NaCl��Һ���Թ��н�����ټ���________�Լ�����Ϻ������ڷ�ˮ�м���5 min�����Թ���ȴ����������Һ��ɫΪ________�����������Թ�����ҺΪ��ɫ�����ȷ����������ΪDNA��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��2010��2011ѧ���Ĵ�ʡ������ѧ�߶���ѧ�ڵ�һ���¿������Ծ� ���ͣ��ۺ���
��11�֣���ͼ��DNA����ijһ�������е��Ұ���ͱ��������ʾ��ͼ�������ش�
��1����ͼ�к���____________�ֺ����___________���Ƿ��ӣ����������������_________����ͼ��ʾ��DNA���Ŵ���Ϣ��_____
��________���̡�
��2����ij����������3��������290����������ɵģ���ô������ҪDNA��________�����������Ƹõ����ʵĺϳɣ������Ҫ__________�ְ����ᡣ
��3���Ҹβ�����һ��Լ��3200��������������ɵ�˫��DNA���������ֲ����ĸ��Ʒ�ʽ��Ϊ���⣬��Ҫ��������ͼ��ʾ����ͬ�ڼ�ͼ������������ [ ]_________���̡�
��4���������ķ����������ϸ���Ŵ���Ϣ�Ĵ��ݹ���________________________________��
��5������DNAʱ��һ���Ƚ���������������ʵ���Ũ��Ϊ0.015mol/L NaCl��Һ���Թ��н�����ټ��� �Լ�����Ϻ������ڷ�ˮ�м���5min�����Թ���ȴ����������Һ��ɫΪ �����������Թ�����ҺΪ��ɫ�����ȷ����������ΪDNA��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010��2011ѧ���Ĵ�ʡ�߶���ѧ�ڵ�һ���¿������Ծ� ���ͣ��ۺ���
��11�֣���ͼ��DNA����ijһ�������е��Ұ���ͱ��������ʾ��ͼ�������ش�

��1����ͼ�к���____________�ֺ����___________���Ƿ��ӣ����������������_________����ͼ��ʾ��DNA���Ŵ���Ϣ��_____
��________���̡�
��2����ij����������3��������290����������ɵģ���ô������ҪDNA��________�����������Ƹõ����ʵĺϳɣ������Ҫ__________�ְ����ᡣ
��3���Ҹβ�����һ��Լ��3200��������������ɵ�˫��DNA���������ֲ����ĸ��Ʒ�ʽ��Ϊ���⣬��Ҫ��������ͼ��ʾ����ͬ�ڼ�ͼ������������ [ ]_________���̡�
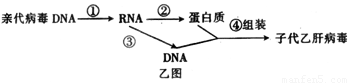
��4���������ķ����������ϸ���Ŵ���Ϣ�Ĵ��ݹ���________________________________��
��5������DNAʱ��һ���Ƚ���������������ʵ���Ũ��Ϊ0.015mol/L NaCl��Һ���Թ��н�����ټ��� �Լ�����Ϻ������ڷ�ˮ�м���5min�����Թ���ȴ����������Һ��ɫΪ �����������Թ�����ҺΪ��ɫ�����ȷ����������ΪDNA��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2012���Ĵ�ʡ��֦���и߶�4���¿������Ծ� ���ͣ��ۺ���
��ͼ��DNA����ijһ�������е��Ұ���ͱ��������ʾ��ͼ�������ش�

��1����ͼ�к���____________�ֺ����___________���Ƿ��ӣ����������������_________����ͼ��ʾ��DNA���Ŵ���Ϣ��_____
��________���̡�
��2����ij����������3��������290����������ɵģ���ô������ҪDNA��________�����������Ƹõ����ʵĺϳɣ������Ҫ__________�ְ����ᡣ
��3���Ҹβ�����һ��Լ��3200��������������ɵ�˫��DNA���������ֲ����ĸ��Ʒ�ʽ��Ϊ���⣬��Ҫ��������ͼ��ʾ����ͬ�ڼ�ͼ������������ [ ]_________���̡�

��4���������ķ����������ϸ���Ŵ���Ϣ�Ĵ��ݹ���________________________________��
��5������DNAʱ�������Ƚ���������������ʵ���Ũ��Ϊ0.015mol/L NaCl��Һ���Թ��н�����ټ��� �Լ�����Ϻ������ڷ�ˮ�м���5min�����Թ���ȴ����������Һ��ɫΪ �����������Թ�����ҺΪ��ɫ�����ȷ����������ΪDNA��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
��ͼ��DNA����ijһ�������е��Ұ���ͱ��������ʾ��ͼ�������ش�
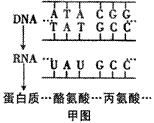
��1����ͼ�к���____________�ֺ����___________���Ƿ��ӣ����������������_________����ͼ��ʾ��DNA���Ŵ���Ϣ��_____
��________���̡�
��2����ij����������3��������290����������ɵģ���ô������ҪDNA��________�����������Ƹõ����ʵĺϳɣ������Ҫ__________�ְ����ᡣ
��3���Ҹβ�����һ��Լ��3200��������������ɵ�˫��DNA���������ֲ����ĸ��Ʒ�ʽ��Ϊ���⣬��Ҫ��������ͼ��ʾ����ͬ�ڼ�ͼ������������ [ ]_________���̡�
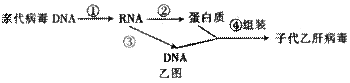
��4���������ķ����������ϸ���Ŵ���Ϣ�Ĵ��ݹ���________________________________��
��5������DNAʱ��һ���Ƚ���������������ʵ���Ũ��Ϊ0.015mol/L NaCl��Һ���Թ��н�����ټ��� �Լ�����Ϻ������ڷ�ˮ�м���5min�����Թ���ȴ����������Һ��ɫΪ �����������Թ�����ҺΪ��ɫ�����ȷ����������ΪDNA��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com