
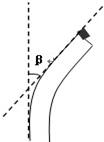
���� ������ԭ����ʹ�õ�����Լ�����Ҫ�������䣻����Լ����ɼ�Һ������Ũ��Ϊ0.1g/mL����������Һ������Һ������Ũ��Ϊ0.05g/mL����ͭ��Һ����ɣ����ڼ�����ԭ�ǣ�ʹ��ʱҪ����Һ����Һ��Ͼ��Ⱥ��ټ��뺬��Ʒ���Թ��У�����ˮԡ���ȣ�˫�����Լ���AҺ������Ũ��Ϊ0.1 g/mL����������Һ����BҺ������Ũ��Ϊ0.01 g/mL����ͭ��Һ����ɣ����ڼ��������ʣ�ʹ��ʱҪ�ȼ�AҺ���ټ���BҺ��
��� �⣺��1��������ԭ����ʹ�õ�����Լ�����Ҫ�������䣻����Լ����ɼ�Һ������Ũ��Ϊ0.1g/mL����������Һ������Һ������Ũ��Ϊ0.05g/mL����ͭ��Һ����ɣ����ڼ�����ԭ�ǣ�ʹ��ʱҪ����Һ����Һ��Ͼ��Ⱥ��ټ��뺬��Ʒ���Թ��У�����ˮԡ���ȣ����ղ���ש��ɫ������
��2��˫�����Լ���AҺ������Ũ��Ϊ0.1 g/mL����������Һ����BҺ������Ũ��Ϊ0.01 g/mL����ͭ��Һ����ɣ����ڼ��������ʣ�ʹ��ʱҪ�ȼ�AҺ���ټ���BҺ��
�ʴ�Ϊ��
��1����� NaOH CuSO4 50��65 ש��ɫ����
��2��˫���� NaOH CuSO4 A
���� ���⿼������Լ���˫�����Լ���ʹ�ñȽϣ����ڿ��鿼������ʵ��Ŀ�ġ�ԭ���������Ͳ������裬������صIJ������ܣ����ܽ���Щʵ���漰�ķ����ͼ��ܽ����ۺ����ã�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | ���˷�˾��ɽ�����������������������Ϊ������������ʱ������ֳ��Ҫ��ʱ�����˿� | |
| B�� | ���ӡ����ߴ�������ȫ�����Ļ����У��ɽ���ʧ��С����С | |
| C�� | �����ɱ���ʱ����ϸ�������������������������CO2����ʹ�������ͷ��� | |
| D�� | ������ѿ�ǡ����ѡ���ʳ�ͽ�ĸ�����ڿ���ͨ��������£����Բ������־� |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
 ��һ��������ѿ�ʵĶ����г����ֱ����������ú��в�ͬŨ�������ص���֬�飬�����ںڰ���12h����ѿ�ʿ���������������ͼ��������ѿ�������ĽǶȦ£����ᣩ����֬����������Ũ�ȣ����ᣩ�Ĺ�ϵ�ɱ�ʾΪ��������
��һ��������ѿ�ʵĶ����г����ֱ����������ú��в�ͬŨ�������ص���֬�飬�����ںڰ���12h����ѿ�ʿ���������������ͼ��������ѿ�������ĽǶȦ£����ᣩ����֬����������Ũ�ȣ����ᣩ�Ĺ�ϵ�ɱ�ʾΪ��������| A�� | 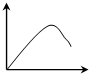 | B�� |  | C�� | 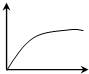 | D�� | 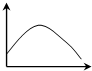 |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢ� | C�� | �٢� | D�� | �ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | Ҷ�������˫��Ĥ���ǽ��й�����õij��� | |
| B�� | ��������е���Ĥ��������������������������Ҫ���� | |
| C�� | ��������Ĥ�������ڶ���ϸ���͵͵�ֲ��ϸ���� | |
| D�� | ��������Ĥ���ǵ����ʺϳɵij��� |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����

| A�� | a��b��c�ֱ���м������ѡ��ܾ����á���˿���� | |
| B�� | OP�ĺ�DNA������MN����1����ԭ����NO�������ܾ����� | |
| C�� | JK����ͬԴȾɫ��������ֿ� | |
| D�� | IJ��MN��Ⱦɫ�������ܲ�ͬ |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
 ͼ��ʾ����һ��ϸ���Ĺ������ǿ�������ǿ��֮���ϵ�����ߣ�����Ϊ�����ĸ�ѡ���У��ܴ�����ϸ��������״̬��������AB��������ǣ�������
ͼ��ʾ����һ��ϸ���Ĺ������ǿ�������ǿ��֮���ϵ�����ߣ�����Ϊ�����ĸ�ѡ���У��ܴ�����ϸ��������״̬��������AB��������ǣ�������| A�� |  | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | �������õ��м�����ͪ�����ͨ��������˫��Ĥ | |
| B�� | �Ƿ����CO2������������������������Ҫ���� | |
| C�� | �ߵ�ֲ��ֻ�ܽ����������������ܽ����������� | |
| D�� | ��ɵ������ڲֿ��в��ܽ��к������� |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | Ѫ����ѹ�Ĵ�С��Ҫȡ����Ѫ���������κ�ˮ�ĺ��� | |
| B�� | ͻ��ǰĤ�ͷŵ����ʿɾ���ɢ����ͨ����֯Һ�壬������ͻ����Ĥ�� | |
| C�� | ����Ѫ��Ũ������ʱ������Ѫ�Ǿͻ�ͨ�������γɵ���Һ�ų����⣬��ά���ڻ�������̬ | |
| D�� | Ѫ�����ס���Һ������������ڻ��������ʳɷ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com