
���������У��е����ڹ�������İ����ᣬ�еIJ��ǣ��������й�������İ��������ϳɻ����������ѡ���ȷ���ǣ� ��
��NH2��CH2��COOH �� NH2��CH2��CH2OH �� NH2��CH2��CH��COOH
|
COOH
�� NH2��CH2��CH��COOH �� NH2��CH2�� CH��NH2
| |
NH2 COOH
A���û����ﺬ��3���ļ� B���û����������
C���û����ﺬ��3������İ��� D���û����ﺬ��1��������Ȼ�
 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
���������У��е����ڹ��ɵ����ʵİ����ᣬ�еIJ��ǡ��������й��ɵ����ʵİ��������ϳɶ��ģ������еİ������Ȼ����ļ�����Ŀ�����ǣ� ��
A.1��1��2 B.3��3��3 C.2��2��2 D.2��2��3
��NH2��CH2��COOH ��NH2��CH2��CH2OH
��![]() ��
��![]() ��
��![]()
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2011-2012ѧ�����ʡ����н�����ѧ�߶�9�·��¿������Ծ� ���ͣ��ۺ���
��10�֣����������У��е����ڹ��ɵ����ʵİ����ᣬ�еIJ��ǡ��������еİ��������ϳ�һ�����ģ���ش��������⣺

|

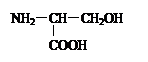
|
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2014��ɽ��ʡ��һ��ѧ�����п��������Ծ� ���ͣ�ѡ����
���������У��е����ڹ���������İ����ᣬ�еIJ��ǡ��������й���������İ����ᣨÿ��һ��������ˮ���Ϸ�Ӧ�γɵĻ������

A.���� B.���� C.���� D.������
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2014���Ĵ�ʡ��һ��ѧ�����п��������Ծ� ���ͣ�ѡ����
���������У��е����ڹ��ɵ����ʵİ����ᣬ�еIJ��ǡ��������й��ɵ����ʵİ��������ϳɶ��ģ������к��еİ������Ȼ����ļ�����Ŀ�����ǣ� ��
��NH2��CH2��COOH ��NH2��CH2��CH2OH �� NH2��CH��CH2��COOH
COOH
�� NH2��CH��CH2��COOH �� NH2��CH��(CH2)4��NH2
NH2 COOH
A��2��2��2 B��3��3��2 C��4��3��3 D��3��4��3
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010�꽭��ʡ��һ��ѧ�����п��������Ծ� ���ͣ�ѡ����
���������У��е����ڹ�������İ����ᣬ�еIJ��ǡ��������й�������İ��������ϳɻ���������к��а������Ȼ����ļ���Ŀ�����ǣ�������

A��3��3��2�� B��2��2��2�� C��3��2��3�� D��3��4��2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com