
ijĶ¬Ń§ĪŖĮĖĢ½¾æĖį¼ī¶Č¶Ō½ĶÄø¾ś¾Ę¾«·¢½ĶĆø“Ł·“Ó¦ĖŁ¶ČµÄÓ°Ļģ£¬Éč¼ĘĮĖŅŌĻĀŹµŃ锣
£Ø1£©ŹµŃéÓĆ¾ß£ŗÉÕ±”¢×¢ÉäĘ÷4Ö§”¢·²ŹæĮÖ”¢øɽĶÄø·Ū”¢ÕįĢĒ”¢ÅäÖĘŗƵÄpH·Ö±šĪŖ5”¢6”¢7”¢8µÄ»ŗ³åŅŗ”£
£Ø2£©ŹµŃé²½Öč£ŗ
¢ŁÓĆæŖĖ®ÅäÖĘÖŹĮæ·ÖŹżĪŖ10£„µÄÕįĢĒČÜŅŗ100ŗĮÉż£¬ĄäČ“µ½ŹŅĪĀ£Ø26”ę£©ŗ󣬼ÓČė4æĖøɽĶÄø·Ū£¬10·ÖÖÓŗó£ŗ
¢Ś______________________________£»
¢Ū______________________________£»
¢Ü______________________________£»
¢Ż______________________________”£
£Ø3£©ŹµŃé¼ĒĀ¼£ŗ²śÉś2ŗĮÉżĘųĢåĢå»żĮæ£ØŅŌÕėĶ²ĶʱśÉĻÉżµÄŗĮÉżŹżĪŖ²śĘųĮ棩ĖłŠčŅŖµÄŹ±¼äČēĻĀ±ķ£ŗ
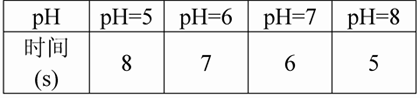
£Ø4£©ŹµŃé½įĀŪ£ŗ______________________________
£Ø5£©ŹµŃé¹ż³ĢÖŠĪŖŹ²Ć“ŅŖµČµ½ÕįĢĒČÜŅŗĄäČ“ŗóŌŁ¼ÓČė½ĶÄø·Ū£æ
___________________________________________ӣ
|
£Ø 2£©¢ŚČ”4֧עÉäĘ÷·Ö±š±źŗÅA”¢B”¢C”¢D¢Ū·Ö±š³éČ” 2ŗĮÉżŗ¬½ĶÄøµÄÕįĢĒČÜŅŗ¢Ü A”¢B”¢C”¢D¶ŌÓ¦³éČ”pHÖµĪŖ5”¢6”¢7”¢8µÄ»ŗ³åŅŗ£¬²¢ÓĆ·²ŹæĮÖĶæÄØŌŚÕėĶ·ŅŌ¼°ÓŠ¹ŲµŲ·½¢Ż¹Ū²ģ²¢¼ĒĀ¼ÕėĶ²×¶±śÉĻÉż 2ŗĮÉżĖłŠčµÄŹ±¼ä£Ø 4£©pHĪŖ7Ź±£¬½ĶÄø¾śµÄ·¢½ĶĖŁ¶Č×īæģ£¬¹żøß»ņ¹żµĶµÄpH¶¼»įÓ°Ļģ½ĶÄø¾śÖŠĆøµÄ»īŠŌ£¬Ó°Ļģ·¢½ĶĖŁ¶Č£Ø 5£©·ĄÖ¹½ĶÄø¾ś±»øßĪĀɱĖĄŅŌ¼°·ĄÖ¹ĆøŹ§Č„»īŠŌ”£½āĪö£ŗøł¾ŻĢāŅā£¬ŌŚÅäÖĘŗĆ 10£„µÄÕįĢĒČÜŅŗŗó£¬ŅŖÓĆøßĪĀĆš¾ś£¬Čē¹ū²»°ŃÕįĢĒČÜŅŗÖŠµÄĻø¾śµČĪ¢ÉśĪļɱĖĄ£¬¾Ķ»įÓ°Ļģ½ĶÄø¾ś·¢½ĶµÄ½į¹ū”£ŌŚĢāÄæÖŠ£¬“żÕįĢĒČÜŅŗ½µĪĀÖĮ26”ꏱ£¬¼ÓČėøɽĶÄø·Ū4æĖ£¬10·ÖÖÓŗó²Å½ųŠŠĻĀŅ»²½ŹµŃéµÄŌŅņŹĒČĆ¼ÓČėµ½ÕįĢĒČÜŅŗÖŠµÄøɽĶÄøÓŠŅ»øö»Öø“»īĮ¦µÄ¹ż³Ģ”£½ÓĻĀĄ“µÄŹµŃé²½Öč¼ū“š°ø£¬µ«ŌŚŹµŃéÖŠŅ»¶ØŅŖ×¢Ņā£ŗŌŚ¼ÓČėÕįĢĒČÜŅŗŗĶ½ĶÄøµÄ»ģŗĻŅŗÓė»ŗ³åŅŗŹ±£¬Ó¦ĻČ¼ÓČė»ŗ³åŅŗ£¬Ź¹½ĶÄø¾śŅ»½ųČėŹŌ¹Ü¾Ķ“¦ÓŚŅ»¶ØµÄpHĢõ¼žĻĀ£¬±ćÓŚŌŚ²»Ķ¬ŹµŃéĢõ¼žĻĀ±Č½Ļ”£ |


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ043
ijĶ¬Ń§ĪŖĮĖĢ½¾æĖį¼ī¶Č¶Ō½ĶÄø¾ś¾Ę¾«·¢½ĶĆø“Ł·“Ó¦ĖŁ¶ČµÄÓ°Ļģ£¬Éč¼ĘĮĖŅŌĻĀŹµŃ锣
£Ø1£©ŹµŃéÓĆ¾ß£ŗÉÕ±”¢×¢ÉäĘ÷4Ö§”¢·²ŹæĮÖ”¢øɽĶÄø·Ū”¢ÕįĢĒ”¢ÅäÖĘŗƵÄpH·Ö±šĪŖ5”¢6”¢7”¢8µÄ»ŗ³åŅŗ”£
£Ø2£©ŹµŃé²½Öč£ŗ
¢ŁÓĆæŖĖ®ÅäÖĘÖŹĮæ·ÖŹżĪŖ10£„µÄÕįĢĒČÜŅŗ100ŗĮÉż£¬ĄäČ“µ½ŹŅĪĀ£Ø26”ę£©ŗ󣬼ÓČė4æĖøɽĶÄø·Ū£¬10·ÖÖÓŗó£ŗ
¢Ś______________________________£»
¢Ū______________________________£»
¢Ü______________________________£»
¢Ż______________________________”£
£Ø3£©ŹµŃé¼ĒĀ¼£ŗ²śÉś2ŗĮÉżĘųĢåĢå»żĮæ£ØŅŌÕėĶ²ĶʱśÉĻÉżµÄŗĮÉżŹżĪŖ²śĘųĮ棩ĖłŠčŅŖµÄŹ±¼äČēĻĀ±ķ£ŗ
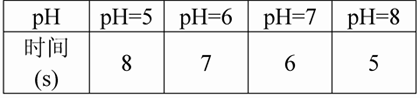
£Ø4£©ŹµŃé½įĀŪ£ŗ______________________________
£Ø5£©ŹµŃé¹ż³ĢÖŠĪŖŹ²Ć“ŅŖµČµ½ÕįĢĒČÜŅŗĄäČ“ŗóŌŁ¼ÓČė½ĶÄø·Ū£æ
___________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijĶ¬Ń§ĪŖĮĖĢ½¾æĖį¼ī¶Č¶Ō½ĶÄø¾ś¾Ę¾«·¢½ĶĆø“Ł·“Ó¦ĖŁĀŹµÄÓ°Ļģ£¬Éč¼ĘĮĖŅŌĻĀŹµŃ锣
£Ø1£©ŹµŃéÓĆ¾ß£ŗÉÕ±”¢×¢ÉäĘ÷4Ö§”¢·²ŹæĮÖ”¢øɽĶÄø·Ū”¢ÕįĢĒ”¢ÅäÖĘŗƵÄpH·Ö±šĪŖ5”¢6”¢7”¢8µÄ»ŗ³åŅŗ”£
£Ø2£©ŹµŃé²½Öč£ŗ
¢ŁÓĆæŖĖ®ÅäÖĘÖŹĮæ·ÖŹżĪŖ10%µÄÕįĢĒČÜŅŗ100ŗĮÉż£¬ĄäČ“µ½ŹŅĪĀ£Ø26 ”ę£©ŗ󣬼ÓČė4æĖøɽĶÄø·Ū£¬10·ÖÖÓŗó£»
¢Ś_____________________________________________£»
¢Ū_____________________________________________£»
¢Ü_____________________________________________£»
¢Ż_____________________________________________”£
£Ø3£©ŹµŃé¼ĒĀ¼£ŗ²śÉś2ŗĮÉżĘųĢåĢå»żĮæ£ØŅŌÕėĶ²ĶʱśÉĻÉżµÄŗĮÉżŹżĪŖ²śĘųĮ棩ĖłŠčŅŖµÄŹ±¼äČēĻĀ±ķ£ŗ
| pH | pH=5 | pH=6 | pH=7 | pH=8 |
| Ź±¼ä£Øs£© | 8 | 7 | 6 | 7.5 |
£Ø4£©ŹµŃé½įĀŪ£ŗ___________________________________________”£
£Ø5£©»Ų“šĪŹĢā£ŗŹµŃé¹ż³ĢÖŠĪŖŹ²Ć“ŅŖµČµ½ÕįĢĒČÜŅŗĄäČ“ŗóŌŁ¼ÓČė½ĶÄø·Ū£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2013-2014ѧğ½ĖÕŹ”ø߶žÉĻѧʌæŖѧ²āŹŌÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ×ŪŗĻĢā
£Ø7·Ö£©ŹµŃéĢ½¾æ£ŗijĶ¬Ń§ĪŖĮĖĢ½¾æĖį¼ī¶Č¶Ō½ĶÄø¾ś¾Ę¾«·¢½ĶµÄĆø“Ł·“Ó¦ĖŁĀŹµÄÓ°Ļģ£¬Éč¼ĘĮĖŅŌĻĀŹµŃé£ŗ
£Ø1£©ŹµŃéÓĆ¾ß£ŗÉÕ±”¢×¢ÉäĘ÷ 4 Ö§”¢·²ŹæĮÖ”¢øɽĶÄø·Ū”¢ĘĻĢŃĢĒ”¢ÅäÖĘŗƵÄpH·Ö±šĪŖ5”¢6”¢7”¢8µÄ»ŗ³åŅŗ”£
£Ø2£©ŹµŃé²½Öč£ŗ
¢ŁÓĆæŖĖ®ÅäÖĘÖŹĮæ·ÖŹżĪŖ 10% µÄĘĻĢŃĢĒČÜŅŗ 100mL £¬ĄäČ“µ½ŹŅĪĀ£Ø 26”ę £©ŗ󣬼ÓČė 4g øɽĶÄø·Ū£¬·“Ó¦10min ”£
¢Ś_______________________________________________________
¢Ū__________________________________________________________
¢Ü____________________________________________________________
¢Ż____________________________________________________________
£Ø3£©ŹµŃé¼ĒĀ¼£ŗ²śÉś 2mL ĘųĢåĢå»żĮæ£ØŅŌÕėĶ²ĶʱśÉĻÉżµÄŗĮÉżŹż±ķŹ¾ĪŖ²śĘųĮ棩ĖłŠčŅŖµÄŹ±¼äČēĻĀ±ķ£ŗ
|
pH |
pH=5 |
pH=6 |
pH=7 |
pH=8 |
|
Ź±¼ä £Ø s £© |
8 |
7 |
6 |
7.5 |
£Ø4£©ŹµŃé½įĀŪ£ŗ________________________________________________________”£
£Ø5£©»Ų“šĪŹĢā£ŗŹµŃé¹ż³ĢÖŠĪŖŹ²Ć“ŅŖµČµ½ĘĻĢŃĢĒČÜŅŗĄäČ“ŗóŌŁ¼ÓČė½ĶÄø·Ū£æ
___________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com