
��.��13�֣���ͼ�Ǵ����ϸ����ij��������ʾ��ͼ���±�������ϸ����ϸ���д��ڵ�����ˮͨ������AQP7��AQP8�IJ��ֱȽϡ�������ش��������⣺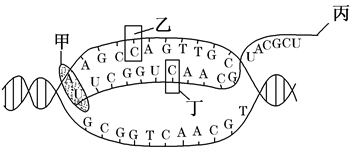
| �Ƚ���Ŀ | AQP7 | AQP8 |
| �������� | 1 | 1 |
| �������� | 269 | 263 |
| mRNA���� | 1500 | 1500 |
| �Թ��������� | ������ | ���� |
| غ���б������ | ����ľ����б��� | ������ĸϸ������ϸ���б��� |
��22�֣�
��.��13�֣�(1)ת¼��1�֣���RNA�ۺ�ø��2�֣� ϸ���ˡ������壨2�֣�
(2)����ऺ��Ǻ����ᣨ2�֣�������ҵĻ�ѧ����֮һΪ�������ǣ�����Ӧ����ɶ��Ļ�ѧ����Ϊ���ǣ�2�֣�
(3)������ѡ���Ա��2�֣�
(4)���������������˲�ͬ�ļӹ����ֱ��г��˲�ͬ�����İ����ᣨ2�֣�
��(9�֣�ÿ��1��)
��ά��ø����ø ��2��ԭ�������ں� �������ͻ�ѧ�����缤/���Ҷ����յ���
��3���߶����� ��4����˿���� ������֯ ��5��A+B (AA+B)/2 ��ˮ����
�������������I����1��ͼʾ�Ĺ�������DNA�ϳ�RNA�Ĺ���Ӧ��ת¼��ת¼��ҪRNA�ۺ�ø����ø��������RNA�ۺ�ø��
��2������RNA�ϵĺ����ᣬӦ�ǰ���ऺ��Ǻ����ᣬ�����ϵ��ǰ�����������Ǻ����ᣬ�����Dz����������ǣ����������Ǻ��ǡ�
��3��ϸ���ֻ���ʵ���ǻ����ѡ���Ա��
��4����AQP7��AQP8�ϳɹ����У���������ϵ���ʹRNA�����Ŀ��ͬ����AQP7��AQP8������������Ŀ��ͬ��ԭ��Ӧ�Ƿ��������������˲�ͬ�ļӹ����ֱ��г��˲�ͬ�����İ����ᡣ
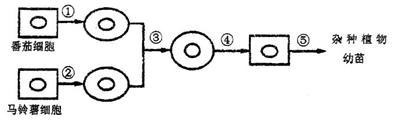
II����1���٢ڹ�����ȥ��ϸ���ڻ��ԭ�����壬��Ҫ����ά��ø����ø������
��2�����̢���ԭ��������ںϣ�������������缤�ͻ�ѧ����PEG�����յ��ںϡ�
��3��ֲ��ϸ���ڵ��γɺ߶�����������أ�����Ҫ�߶�������롣
��4����Ҫ�����ѷֻ��γ�������֯���ٷֻ��γ����磬�ù������漰�ĵ��ķ��ѷ�ʽ������˿���ѡ�
��5������-��������ֱ�ӽ�ϸ���ں�����һ�𣬹ʺ���Ⱦɫ����Ŀ��A+B����������ӽ����ַ����ܳɹ��Ļ�����������A/2��B/2�ܾ��õ��ĺ����Ӧ����A+B��/2��Ⱦɫ�壬������û��ͬԴȾɫ�岻��������Ҫʹ�ӽ�����ָ����ԣ���������ˮ���ض��������Ⱦɫ����Ŀ�ӱ�������
���㣺���⿼���������ϸ���������֪ʶ�����ڿ��쿼����֪ʶ���ʶ������Ͷ�ͼ�η���������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ͼl��ʾ��̬ϵͳ�и��ɷ�֮�����ϵ��ͼ2Ϊһ��ʱ����ijһ��̬ϵͳ�еļ�����Ⱥ�����仯���ߣ����ͼ�ش�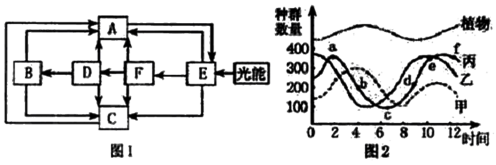
��1����ͼ1�ĸ�����ĸ�У������빹��Ⱥ����� ������ĸ����
��2��ͼl����������ѭ������Ҫ������ ������ĸ�ͼ�ͷ��ʾ����
��3��F�ķ�㱻�ֽ��߷ֽ⣬Ҳ���DZ�ʾ ������ĸ��ͬ�����������ֽ������á�
��4��ij��������Ⱦ���E�г��ֲ��ְ��磬��F��D������Ӱ����F ��D ��
��5��ͼ2�б�����bc���½���ԭ�� ��
��6����ͼl��һũҵ��̬ϵͳ��ijУ�����о���ѧϰС�������п��췢�֣��ոѵ���ȼ��ʹ�ã���㡢��ˮ��Ϊ����ֱ��ʩ��ũ��ɴ����
��2�֣������2�㣩�Ȳ�������������һ����С�����йز��Ž��飬���ýո�����������ά�ع���Ĥ��Ϊ��Ĥʹ�ã�����һ���̶��Ͻ�����ֵ����⡣��Ĥ������Ȼ��������ͼ1�е� ������ĸ�������� �����⡣
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
Ӧ�����﹤�̼������������Ҫ��������Ʒ�ֻ��²�Ʒ���������ѧ����ת��Ϊ����������Ҫ���֡����ͼ�ش��������⣺ 
��1��������ת����ɽ��Ĺ����У��ٹ�����Ҫ�Ĺ���ø�� �� ���ڹ��̳��õķ����� ��
��2��ת��Ѫ˨����ɽ�����ͨ��������֭��������Ѫ˨ҩ��ڹ�������Ӧ������������У��˿�Ѫ˨������˱��뺬��ʹ�������ɽ������ϸ���������Ա����___________������__________________ʶ��ͽ�ϵ㲿λ������ͨ��������ת�������Ϊ ��
��3��ɽ��������̥����ֲ������ĸ���ӹ�֮ǰ���������̥�����Ա������һ����ȡ����_________________ʱ�ڵ�____________ϸ�����м�⡣��Ҫ��������ֻ��ͬ��ת��Ѫ˨����ɽ���Բ��� ������ȡ��
��4��prG����IJ����ܼ���ϸ�����Ϸ��ѣ�ͨ�����̵���õ��ػ������Ʊ�����¡���壬���������
ϸ�����������ϸ������ ���ص㡣
��5�����������������У�ͨ������Ti���������������������ϸ����Ϊ��ʹ�������˳�����ӵ�����Ⱦɫ��������У��������ͨ��λ��Ti������____________Ƭ���С��ӷ���ˮƽ��⿹�����Ƿ������ɹ��ķ�����________________________���Ӹ���ˮƽ��⿹�����Ƿ������ɹ��ķ�����________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��9�֣���з��ɫ������������Ұ�ɫ����ɫ�ͻ���ɫ����������Ӧԭ����ͼ��ʾ������A���ƺϳ�ø1������B���ƺϳ�ø2������b���ƺϳ�ø3������a���ƺϳɵĵ�������ø1���ԣ�����a���Ϻ����ʼף��������ࣩ�����ڹ�����ۣ����³������50%�����������ʻ��۱���Ϊ�Ұ�ɫ�ǣ������ʻ��۱���Ϊ��ɫ�ǣ������ʻ��۱���Ϊ����ɫ�ǡ���ش�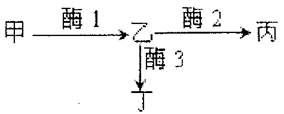
��1����з����ɫ������ �Ի�����Ƶġ���ɫ����з�Ļ������� �֣��ֱ��� ��
��2����ֻ��ɫ����з���䣬�������ֻ�лҰ�ɫ��з����ɫ��з���ұ���Ϊ1��6���ױ����������
Ϊ �� ��
��3��������ΪAaBb����ֻ��з�ӽ�������ij������Ϊ �������Ϊ ��
��4��������ʵ����Կ���������ͨ������ �����ƴ�����̣������������������״��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
����ķ�ֳ����ڴ�����У�����ʱ��Ϊһ��������ڡ���ͼΪij��������һ�������ڵļ��ص��ڹ��̣����ͼ�����ش�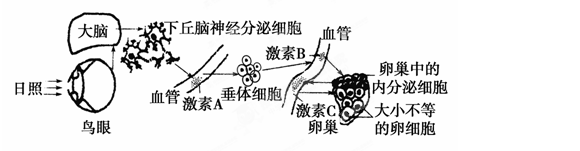
(1)��ͼʾ��Ϣ��֪�����ֳ��Ϊ�ĵ��ڷ�ʽΪ _______________________��
(2)����ϸ���ı������Ƿ����ʶ����C�����壿____________��������__________________________��
(3��ͼ�жϣ���Ҫ��֤B����������________���ܡ����ܣ���ȥ�����ٵ�����Ϊʵ�鶯���ԭ����__________________________________________________________________��
(4)���������ע�伤��C���Լ���A�ͼ���B��Ӱ��ֱ���_________��___________��
(5)�������������ʹ�����������ͼʾ��ϢӦ��ȡ�Ĵ�ʩ��_______________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
���ܲ��ؿ���Ԥ�������ϡ������ڱ����۾��������ά���֣������Դٽ����ڸй�ɫ�ص����ɣ���ǿ�������γɣ�������Ԥ��ҹä֢����ǿ�۾���ɫ�Ĺ��ܡ����������ȡ���̼����ܲ��ص����ʣ��ش��������⡣
��1�����ܲ����� ɫ�ᾧ���Ӻ��ܲ�����ȡ���ܲ��أ����� ��Ϊ�ܼ���ԭ���� ��
��2���Ӻ��ܲ�������ȡ���ܲ��س��õķ����� ���������ַ�����ȡ���ܲ��ص���Ҫ�����ǣ����顢 �� �����ˡ�Ũ����
��3���ں��ܲ������ļ��ȸ�������У�Ӧ�ϸ� �� ������һ����Χ��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
����dz��õ�����ѧʵ�����,������ͼ�ش��������:
| | ʵ������ | ȡ�IJ�λ | �۲췽ʽ | ���� | ϸ����̬ |
| һ | �۲�ֲ��ϸ�� | ��ƬҶ���Ƥ | A | �߱��� | ������ |
| �� | �۲�ֲ��ϸ�����ʱڷ����븴ԭ | B | ԭɫ�۲� | �ͱ��� | ������ |
| �� | �۲�ֲ��ϸ����˿���� | ��������� | Ⱦɫ�۲� | �߱��� | C |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��������ѿ��������ͼ�������ٷ��ڰ����������ѿ�ʼ�˵��²���������ס�����ڼ�˺��������ĸƬ�����ڼ�˺��������֬Ƭ������ȥ��ѿ�ʵļ�ˣ�����ȥ��ѿ�ʼ�ˣ������п��Ϸ�һ����һ��Ũ�������ص���֬С�飻����ȥ��ˣ����п��Ϸ�һ����һ����ȵ������ص���֬С�鲢�����ڰ��������ȥ��ѿ�ʼ�ˣ����ڰ��������װ�þ�����ͬ�ĵ�������䡣����ϸ����ͼ���ش��������⣺
(1)�е��������ʱ����ֱ����������__________��
(2)�����Դ��������________��
(3)������������������_________��
(4)��Ѣܷ�������ת���ϣ������Ҳ���գ��������������_______________��
(5)�������������ԭ���� ��
(6)����ʵ��˵����ֲ��ľ�����________�����ܹ�̼��IJ�λ��_______��������������IJ�λ��____����ѿ�ʼ���ܹ�����_____���Ӽ�˵��²��������________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
Ϊ̽���ζ�ij����ȼ���������������õ�Ӱ�죬�ڲ�ͬŨ��NaCl�����£����侻������ʡ�����CO2Ũ�ȡ����ɫ�غ����Ƚ��вⶨ���������ͼ������ڼ�ϸ���ĺ���ǿ��û�������仯�������ͼ�ش��������⣺
(1)Ҷ������ɫ�صĹ�����__________________________________________��
(2)�����е�CO2��ͨ��ֲ��ҶƬ����� _________________________________
����ֲ�����ڡ�������ò������л���(C6H12O6)�е�����Դ��ԭ���е� ���л���(C6H12O6)�е�����ϸ�������������ղ���______________ �С�
(3)��NaClŨ����200��250 mmol/Lʱ��������������½�����Ȼ�����¸�ֲ�����ļ����ʵ����羻�������Ҳ������½�������ǰ����Ҫ������ ______
��������Ҫ������__________________________________��
(4)�ܹ�����ʿ��õ�λʱ���ڵ�λҶ����� ��ʾ������Ƹ�ʵ�����ܹ�����ʱ仯���Ƶ�����ͼ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com