
ֲ��������������Ҫ����������ˮ�ֺͿ���Ԫ�����ӡ���ͼ��ʾ��ʩ�ʺ�ĸ����������Ƥϸ����������Һ�е�״̬�����ͼ�ش�
(1)�ò�λ�ı�Ƥϸ���빦������Ӧ�Ľṹ�ص���________________________��
________________________��
(2)ͼ�и���Ƥϸ����ʱ����������״̬��________________________________����ʱ����������ˮ�ֺͿ���������
________________________________________________________________________��
(3)���ṩ0.3 g/mL��������Һ��1 mol/L���������Һ��1 mol/L�Ĵ�����Һ����ҩƷ(������Һ��Ũ�Ⱦ���һ��ϸ��ҺŨ�ȴ�)����ɫ��С�����������ѡ��һ����ȷ��ҩƷ��������Ҫ�������þߣ����һ����ʵ�飬֤ʵ��Ե�(2)��ڶ��ʵ��жϡ�
ʵ�鲽�裺��ȡһ�ɾ����ز�Ƭ�����������1��__________________________����˺ȡ��ɫ�����ƬҶ��Ƥ��ƽչ��Һ���У����ϸDz�Ƭ�����������¹۲졣
ʵ������ϸ���ȳ���__________��������__________��
ʵ����ۣ��ɴ�֤����
________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��2011-2012ѧ�����ʡ�����еڶ�����ѧ��һ��һѧ�ڵڶ����¿������Ծ� ���ͣ��ۺ���
��ÿ��1�֣���8�֣�ֲ��������������Ҫ����������ˮ�ֺͿ���Ԫ�����ӡ���ͼ��ʾ��ʩ�ʺ�ĸ����������Ƥϸ����������Һ�е�״̬�����ͼ�ش�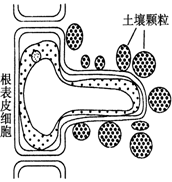
(1)ˮ��ϸ������������ʽ���ڣ�ũ�����ջ�����ӷ��ڳ�Ժɹ��Ϊ�˳�ȥ����_______�������ɹ�����������û�ʧȥ����______ˮ��
(2)ͼ�и���Ƥϸ����ʱ����������״̬��_____����ʱ��������ˮ�ֺͿ��������� ��
(3)���ṩ0.3 g/mL��������Һ��1 mol/L���������Һ��1 mol/L�Ĵ�����Һ����ҩƷ(������Һ��Ũ�Ⱦ���һ��ϸ��ҺŨ�ȴ�)����ɫ��У�����������ѡ��һ����ȷ��ҩƷ��������Ҫ�������þߣ����һ����ʵ�飬֤ʵ��Ե�(2)��ڶ��ʵ��жϡ�
ʵ�鲽�裺
��ȡһ�ɾ����ز�Ƭ�����������1��__________��
��˺ȡ��ɫ�����ƬҶ��Ƥ��ƽչ��Һ���У����ϸDz�Ƭ�����������¹۲졣
ʵ������ϸ���ȳ���________��������________��
ʵ����ۣ��ɴ�֤����_____________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2014�����ʡ�����и�һ��һѧ�ڵڶ����¿������Ծ� ���ͣ��ۺ���
��ÿ��1�֣���8�֣�ֲ��������������Ҫ����������ˮ�ֺͿ���Ԫ�����ӡ���ͼ��ʾ��ʩ�ʺ�ĸ����������Ƥϸ����������Һ�е�״̬�����ͼ�ش�

(1)ˮ��ϸ������������ʽ���ڣ�ũ�����ջ�����ӷ��ڳ�Ժɹ��Ϊ�˳�ȥ����_______�������ɹ�����������û�ʧȥ����______ˮ��
(2)ͼ�и���Ƥϸ����ʱ����������״̬��_____����ʱ��������ˮ�ֺͿ��������� ��
(3)���ṩ0.3 g/mL��������Һ��1 mol/L���������Һ��1 mol/L�Ĵ�����Һ����ҩƷ(������Һ��Ũ�Ⱦ���һ��ϸ��ҺŨ�ȴ�)����ɫ��У�����������ѡ��һ����ȷ��ҩƷ��������Ҫ�������þߣ����һ����ʵ�飬֤ʵ��Ե�(2)��ڶ��ʵ��жϡ�
ʵ�鲽�裺
��ȡһ�ɾ����ز�Ƭ�����������1��__________��
��˺ȡ��ɫ�����ƬҶ��Ƥ��ƽչ��Һ���У����ϸDz�Ƭ�����������¹۲졣
ʵ������ϸ���ȳ���________��������________��
ʵ����ۣ��ɴ�֤����_____________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ��߿���ϰ�½�С��ϰ������һ4-1���ʿ�Ĥ�����ʵ�� ���ͣ��ۺ���
ֲ��������������Ҫ����������ˮ�ֺͿ���Ԫ�����ӡ���ͼ��ʾ��ʩ�ʺ�ĸ����������Ƥϸ����������Һ�е�״̬�����ͼ�ش�

(1)�ò�λ�ı�Ƥϸ���빦������Ӧ�Ľṹ�ص���________________________��
________________________��
(2)ͼ�и���Ƥϸ����ʱ����������״̬��________________________________����ʱ����������ˮ�ֺͿ���������
________________________________________________________________________��
(3)���ṩ0.3 g/mL��������Һ��1 mol/L���������Һ��1 mol/L�Ĵ�����Һ����ҩƷ(������Һ��Ũ�Ⱦ���һ��ϸ��ҺŨ�ȴ�)����ɫ��С�����������ѡ��һ����ȷ��ҩƷ��������Ҫ�������þߣ����һ����ʵ�飬֤ʵ��Ե�(2)��ڶ��ʵ��жϡ�
ʵ�鲽�裺��ȡһ�ɾ����ز�Ƭ�����������1��__________________________����˺ȡ��ɫ�����ƬҶ��Ƥ��ƽչ��Һ���У����ϸDz�Ƭ�����������¹۲졣
ʵ������ϸ���ȳ���__________��������__________��
ʵ����ۣ��ɴ�֤����
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
ֲ��������������Ҫ����������ˮ�ֺͿ���Ԫ�����ӡ���ͼ��ʾ��ʩ�ʺ�ĸ����������Ƥϸ����������Һ�е�״̬�����ͼ�ش�

(1)�ò�λ�ı�Ƥϸ���빦������Ӧ�Ľṹ�ص���________________________��
________________________��
(2)ͼ�и���Ƥϸ����ʱ����������״̬��________________________________����ʱ����������ˮ�ֺͿ���������
________________________________________________________________________��
(3)���ṩ0.3 g/mL��������Һ��1 mol/L���������Һ��1 mol/L�Ĵ�����Һ����ҩƷ(������Һ��Ũ�Ⱦ���һ��ϸ��ҺŨ�ȴ�)����ɫ��С�����������ѡ��һ����ȷ��ҩƷ��������Ҫ�������þߣ����һ����ʵ�飬֤ʵ��Ե�(2)��ڶ��ʵ��жϡ�
ʵ�鲽�裺��ȡһ�ɾ����ز�Ƭ�����������1��__________________________����˺ȡ��ɫ�����ƬҶ��Ƥ��ƽչ��Һ���У����ϸDz�Ƭ�����������¹۲졣
ʵ������ϸ���ȳ���__________��������__________��
ʵ����ۣ��ɴ�֤����
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com