
������ֲ��ϸ�������ṹʾ��ͼ����ش𣺣���13�֣�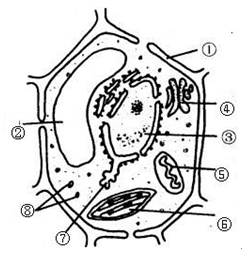
����������д��ţ������������֣����磺���ڣ�ϸ���ˣ�
��1��ͼ������������йص�ϸ�����ǣ� ���ߣߣߣߣߣߣ��ڣߣߣߣߣߣ��ں���������ߣߣߣߣߺ����йص�ø��
��2�����ڷֲ�����ɱ�����Ⱦ��Ⱦ����ɫ�����ʡ���������Ҫ�ɣߣߣߣߣߣߺͣߣߣߣߣߣߣ���ɡ�
��3����������ģߣߣߣߣߣߣ�����ϸ���ϳɣߣߣߣߣߣߣߣߵij�����
��4��ϸ��Ĥ�ķ��ӽṹ���� �� ���ɵģ��ӽṹ�Ͼ��Уߣߣߣߣߣߣ��ԣ��ӹ����Ͽ������ǣߣߣߣߣߣ�Ĥ��
��5��ϸ���ھ���˫��Ĥ�ṹ���У� ���ߣߣߣߣߣߡ��� ���ߣߣߣߣߣߣߡ��� ���ߣߣߣߣߣߣߡ�
��6��ϸ���ڵ����ʵ����������ͨ���ǣ� ���ߣߣߣߣߣߣ�
��7���ڶ�ֲ��ϸ���ж��У�����ֲ��ϸ������ϸ�����γ��йص�ϸ�����ǣ� ���ߣߣߣߣߣߣߡ�
��8��ͼ����Щϸ�����к���DNA�� ���ߣߣߣߣߣߡ��� ���ߣߣߣߣߣߣߡ�
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ���ຣʡ�ຣʦ����2010-2011ѧ��߶���ѧ�����п����������� ���ͣ�071
������ֲ��ϸ�������ṹʾ��ͼ����ش�
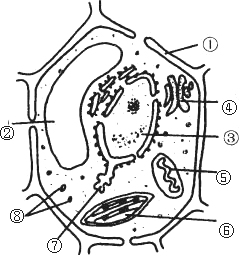
����������д��ţ������������֣����磺���ڣ�ϸ���ˣ�
��1��ͼ������������йص�ϸ�����ǣ�������________����________�ں���������________�����йص�ø��
��2�����ڷֲ�����ɱ�����Ⱦ��Ⱦ����ɫ�����ʡ���������Ҫ��________��________��ɡ�
��3�����������________������ϸ���ϳ�________�ij�����
��4��ϸ��Ĥ�ķ��ӽṹ����_________��________���ɵģ��ӽṹ�Ͼ���________�ԣ��ӹ����Ͽ�������________Ĥ��
��5��ϸ���ھ���˫��Ĥ�ṹ���У�������________����������________����������________��
��6��ϸ���ڵ����ʵ����������ͨ���ǣ�������________
��7���ڶ�ֲ��ϸ���ж��У�����ֲ��ϸ������ϸ�����γ��йص�ϸ�����ǣ�������________��
��8��ͼ����Щϸ�����к���DNA��������________����������________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2014��ɽ��ʡ��һ�������¿������Ծ� ���ͣ��ۺ���
������ijϸ�������ṹģʽͼ���ش�(���������ţ�������������)

��1����ͼ��_ ϸ�������ṹʾ��ͼ��
��2���ṩϸ�������ġ�����������Ϊ[ ]________________��
��3�����빹������Ĥϵͳ��ϸ������[ ]
��4�����ü��̡�������Ⱦɫ����ϸ��Ⱦɫ���������¿����������� ��
��5��Ϊ�о�ϸ���ڸ�����ɳɷֺ��ܣ��轫ϸ�������롣�������ϸ�������õķ����� ��
��6��Ҷ��������ʵ����ɫ������ɫ�⣬������ɫ����[ ] �е�ɫ����������
��7������ϸ�����ͼ��ʾ��ϸ���Ƚϣ������������ ��
��8������ϸ��Ϊ�����������ϸ������ͼ�в�Ӧ���ֵ�ϸ����[ ]��
(9) �ṹ��Ϊ_____________���ڶ���ϸ���еĹ���Ϊ______________________����ֲ��ϸ����˿����ʱ����_______________�γ��йء�
(10) ������������___________________.���Խ�________________ת��Ϊ��ѧ�ܡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010���ຣʡ�߶���ѧ�����п��������Ծ� ���ͣ��ۺ���
������ֲ��ϸ�������ṹʾ��ͼ����ش𣺣���13�֣�
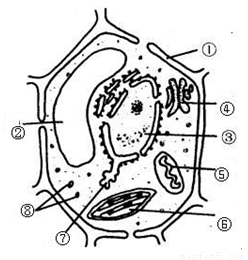
����������д��ţ������������֣����磺���ڣ�ϸ���ˣ�
��1��ͼ������������йص�ϸ�����ǣ� ���ߣߣߣߣߣߣ��ڣߣߣߣߣߣ��ں���������ߣߣߣߣߺ����йص�ø��
��2�����ڷֲ�����ɱ�����Ⱦ��Ⱦ����ɫ�����ʡ���������Ҫ�ɣߣߣߣߣߣߺͣߣߣߣߣߣߣ���ɡ�
��3����������ģߣߣߣߣߣߣ�����ϸ���ϳɣߣߣߣߣߣߣߣߵij�����
��4��ϸ��Ĥ�ķ��ӽṹ���� �� ���ɵģ��ӽṹ�Ͼ��Уߣߣߣߣߣߣ��ԣ��ӹ����Ͽ������ǣߣߣߣߣߣ�Ĥ��
��5��ϸ���ھ���˫��Ĥ�ṹ���У� ���ߣߣߣߣߣߡ��� ���ߣߣߣߣߣߣߡ��� ���ߣߣߣߣߣߣߡ�
��6��ϸ���ڵ����ʵ����������ͨ���ǣ� ���ߣߣߣߣߣߣ�
��7���ڶ�ֲ��ϸ���ж��У�����ֲ��ϸ������ϸ�����γ��йص�ϸ�����ǣ� ���ߣߣߣߣߣߣߡ�
��8��ͼ����Щϸ�����к���DNA�� ���ߣߣߣߣߣߡ��� ���ߣߣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
������ֲ��ϸ�������ṹʾ��ͼ����ش𣺣���13�֣�

����������д��ţ������������֣����磺���ڣ�ϸ���ˣ�
��1��ͼ������������йص�ϸ�����ǣ� ���ߣߣߣߣߣߣ��ڣߣߣߣߣߣ��ں���������ߣߣߣߣߺ����йص�ø��
��2�����ڷֲ�����ɱ�����Ⱦ��Ⱦ����ɫ�����ʡ���������Ҫ�ɣߣߣߣߣߣߺͣߣߣߣߣߣߣ���ɡ�
��3����������ģߣߣߣߣߣߣ�����ϸ���ϳɣߣߣߣߣߣߣߣߵij�����
��4��ϸ��Ĥ�ķ��ӽṹ���� �� ���ɵģ��ӽṹ�Ͼ��Уߣߣߣߣߣߣ��ԣ��ӹ����Ͽ������ǣߣߣߣߣߣ�Ĥ��
��5��ϸ���ھ���˫��Ĥ�ṹ���У� ���ߣߣߣߣߣߡ��� ���ߣߣߣߣߣߣߡ��� ���ߣߣߣߣߣߣߡ�
��6��ϸ���ڵ����ʵ����������ͨ���ǣ� ���ߣߣߣߣߣߣ�
��7���ڶ�ֲ��ϸ���ж��У�����ֲ��ϸ������ϸ�����γ��йص�ϸ�����ǣ� ���ߣߣߣߣߣߣߡ�
��8��ͼ����Щϸ�����к���DNA�� ���ߣߣߣߣߣߡ��� ���ߣߣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com