
��7�֣���ͼ�ױ�ʾij�������ú���������ˮ�Ļ���ԭ����ͼ�ұ�ʾ��ˮ��A�����������ͬ�ط����ֳɷ���Ժ����ı仯����������ѧ֪ʶ�������ش��������⣺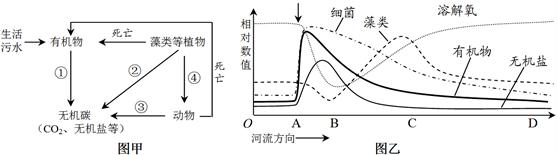
��1��ͼ������ɹ��̢ٵ�����������̬ϵͳ�ɷ��е� ���ú�����̬ϵͳ������������Դ�� �����̢ں͢۱�ʾ������������ ��
��2��ͼ����AB���ܽ����Ľ�����Ҫԭ���� ��BC�����������ֳ����Ҫԭ���� ��
��3�����ŷŽ������IJ���������ˮ�����ǻ��ʳ�����ˮ����������������ӵ����������� ��
��4����ͼ�Ģ��д��ڡ���������ζ�������������㡱ʳ����������n��Ӫ����������ΪakJʱ�����m��m>n����Ӫ����������������ֵy����ѧģ�Ϳɱ�ʾΪ��
| A��y=a��10n-m | B��y=a��10m | C��y=a��5n-m | D��y=a��5m-n |
��1���ֽ��ߣ�1�֣�
�����ֲ��Ĺ�����ú�������ˮ�е��л��1�֣�
�������ã���ϸ��������������������1�֣�
��2��ϸ���ֽ���ˮ�е��л���ʱ���Ĵ�����������1�֣�
ϸ���ֽ��л���������������࣬����������������1�֣�
��3�����ࣨ1�֣�
��4��C��1�֣�
�������������
��1��ͼ���ж�ֲ�������е��л��ﱻ�ֽ�Ϊ������ڷֽ��ߵ����á��ú����������������Դ������ֲ��Ĺ�����ú�������ˮ���ŷţ���ֲ��ͨ�����������ܰ��л���ֽ�����
��2��ͼ����AB���ܽ������͵�ԭ��������ֲ��ļ��ٺ�ϸ���ֽ��л�������������BC�����������ֳ��ԭ����ϸ����ˮ�е��л���ֽ���������ࡣ
��3�����ʳ�����ˮ�е�������Ҫ�����Σ�������������������ֲ�
��4��ÿ����һ��Ӫ������ռ�е��������ֵ�ͱ�Ϊ��һӪ������1/5����5��1�����Ե�m��Ӫ������������������ֵΪy=a��5n-m��
���㣺���⿼����̬�����ı��������ڿ��鿼��������ѧ֪ʶ��Ҫ�㣬����֪ʶ���������ϵ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
(11��)��ͼ1��ʾA.B����ֲ�������������ǿ�ȸı�ı仯���ߣ�ͼ2��ʾ��Aֲ����ڲ�ͬŨ��CO2���������£�Aֲ���������ܹ���ǿ��Ӱ��ı仯���ߡ�
������ش�
(1)�ڽϳ�ʱ����������Ļ����У������ܵ�����Ӱ���ֲ����________________��
(2)ͼ1�еġ�a�����ʾ______________�������ȱþ��ȫӪ��Һ����Aֲ�����磬��b����ƶ�������______________��
(3)��c��ʱ��Ҷ������ADP���ƶ������Ǵ�________________��______________�����ƶ���
(4)e����d����Ƚϣ�e��ʱҶ��ϸ��C3�ĺ���______________��e����f����Ƚϣ�e��ʱҶ��ϸ����C3�ĺ���______________��(��ߡ������͡�����һ�¡�)
(5)������ǿ��Ϊgʱ���Ƚ�ֲ��A.B���л����������M1��M2�Ĵ�С���л���ϳ�����N1��N2�Ĵ�С�����Ӧ�ֱ�ΪM1__________M2��N1__________N2��
(6)��ʩũ�ҷʿ�����߹��Ч�ʵ�ԭ����(��������)��
��__________________________________________________________��
��__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
(10��)�ش�������������ص����⡣
��1����������������Ҫ���� �����3������
��2����ָ���ⴥ����������ֲ�Ƥ�����ף�����ΪƤ��ëϸѪ�����ź�ͨ�����ӣ� ������֯��϶Һ����ۡ�����ָ�˿ڸ�Ⱦ����������Һ������ϸ����ɱ�����ʵ��������ֺ����˹������� ���ߡ�
��3����֢���������������߲����䲡���ǻ�������ϵͳ��ͻ����Ĥ�ϵ��������嵱�� ����������Ӧ���塣�ÿ������������������Խ�ϣ�������ʲ�����������������ϣ��Ӷ����� �ź��� �źŵ�ת���������衣
��4���ٴ���������֢���������ضȻ��ߣ��ɲ��������г�����Ŀ��������_________������Tϸ����Tϸ�����ܲ����ܰ����ӣ��������� ���ϸ�����ߡ�����Һ���ߡ�����Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
ʵ��̽������øϴ�·۵�ʹ��������Ч����ʵ��Ŀ�ģ�
(1)�۲��øϴ�·���ϴ���е�Ч����
(2)̽����øϴ�·۵�����¶�������
ʵ���þ����ϣ�3��100 mL������ƿ�����ӡ��������ˮԡ�������ɿ�մ�м�Ѫ�ҷ���һ����������ּ�øϴ�·ۼ�δ��ø��ϴ�·ۡ�
��Ҫʵ�鲽�裺
��һ�������������ˮԡ�ֱ����40���80�档
�ڶ�������9������ƿ�и���ˮ100 mL��������ƿ�ֳ�A��B��C���飬ÿ����������A���ƿ�м���һ�ּ�øϴ�·�0.5 g����B���ƿ�м�����һ�ּ�øϴ�·�0.5 g����________��
����������ÿ���3������ƿ�ֱ����20�������¡�40���80��ˮԡ�У�����һ��ʱ�䡣
���IJ�����________��
�Իش��������⣺
(1)�Բ�ȫ����ʵ�鲽�衣
��___________________________________________________
_____________________________________________________��
��___________________________________________________
____________________________________________________��
(2)ʵ��������C������ƿ��������_____________________
___________________________________________________��
(3)���������������ϡ����ա��ijɷַ�������øϴ�·��е�ø������________________________��
(4)�������ø�����ص���¶���ø���Թ�ϵ�����⣬��Ϊ��øϴ�·۱�д��ʹ��ָ�ϡ�(����д������)��________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��9�֣�ɽ����ӵ������ɳ�Ⱦɫ���ϵ�λ����A(a)����������A��a�ֱ��Ӧ�к��Ӻ����ӣ�����A��������Ϊ���ԣ��ڴ�����Ϊ���ԡ�ɽ���״������ȱ�����ɵ�λ����B(b)���Ƶij�Ⱦɫ���Ŵ�����һֻ�к��ӹ����6ֻ�������ӵ�ĸ���䣬����6ֻС������1ֻ����ĸ��1ֻ�к���ĸ��3ֻ�к��ӹ���1ֻ���ӡ���״������ȱ�ݵĹ���ͬ���������⣺
(1)ɽ���״������ȱ�����ڳ�Ⱦɫ���ϵ�______���ԡ��������Ŵ��������Ƹü����ĵ�λ����B(b)���λ����A(a)�����______���ڡ����ڣ�ͬһ��ͬԴȾɫ���ϡ������Ŵ�ͼ��˵���������ۣ�
(2)�ױ�ĸ����������______ֻ������ΪAa��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
�ش����й��������ø�����⡣
������Ⱦ�����������Խ��⣬�о���������������ڵ�����ˮ��ø�Ǵ������������Ĺؼ�ø��
(1)���������������������������������________________________________________________________________________��
ԭ����________________________________________________________________________��
A������(g/L)��ij��������2.0��(NH4)2SO42.0��K2HPO43.0��MgSO41.0��pH 7.4���������50 mL
B������(g/L)��ţ���10.0��������20.0��������20.0��NaCl 5.0��pH 7.4
C������(g/L)��ij��������2.0������20.0��NH4NO32.5��MgCl20.5��K2HPO43.0���������50 mL��pH 7.4
��һ���о����֣���ͬ�Ľ������Ӷ�����ˮ��ø�Ļ�����һ��Ӱ�죬������±���
| ��������(mmoL/L) | ��Ի���(%) |
| ������ | 100 |
| Mn2�� | 123 |
| Co2�� | 79 |
| Mg2�� | 74 |

�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
(10��)��ͼ�ױ�ʾ���仡��������Ԫ������ϵ�����С�O��(��ʾ����ͻ�����壬�ٵ���ͻ��ĩ��(��һ����������Ԫģʽ)��ͼ�ұ�ʾͻ���������ṹģʽͼ����ϵͼ��ش��������⣺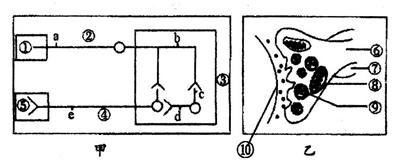
(1)ͼ���У����ٴ���С���ϵĸ����������(������ĩ�Ҽ���֧������ϼ���)��Ϊ_________���۳�Ϊ____________________��
(2)ͼ���д̼�d�㣬���d���⣬ͼ�з����˷ܵĵ㻹��____________________(����ĸ��ʾ)��
(3)ͼ���ж�����̼Ũ����ߴ���[ ]________________�У��ýṹ��������Ϊ���˷ܵĴ����ṩ___________��
(4)���������У�ʹ��ij�־ֲ�����������ʹ��ͼ��[��]______________���ͷŵ�___________����������[ ]______________���Ӷ���ʱʧȥ�˷ܴ������ܡ�
(5)�˷�ͨ��ͼ�ҵĴ��ݹ����У��źŵı仯�����__________________________�����ݷ�����ص���___________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
(15��)��ͼ��ʾ���ۡ�֬���������ʱ����������������ֽ��л�仯��ϵģʽͼ��ͼ����ĸA��B��C��D��E��F��ʾijЩ���ӡ�ԭ�ӻ���ţ�X����ijϸ���������ֱ�ʾ��Ӧ���������̡����ͼ�ش�
1.��д�������������ƣ�
A C D E F
2����д�����������������ƣ�
�� ��
ˮ�IJ���������X�ṹ �� ��λ������ATP�����ǹ��� ��
3�������ǡ������ʡ�֬�����������ֽ�ʱ�����ͷ������⣬���γ���ͬ�Ĵ�л�ղ���______________��
4�������Ѱ������õĹ�����[ ]��
5������һ����______________����½��й��̢ޢݣ���������������幩��������ʱ������C�������ֽ���� ��
6.д�����������ķ�Ӧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
����ֻ�ܴ�����ͻ��С��� �У�ֻ���� �ͷ������� �������Ԫ֮��Ĵ���ֻ���� �ġ��������������� �����ƺ����������� ,ά������ƽ��������� �� ���������еĸ����ࡣ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com