
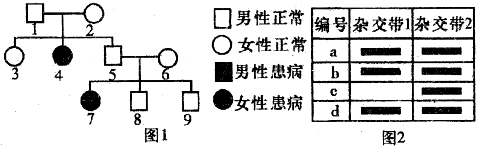
| A£® | Ķ¼2ÖŠµÄ±ąŗÅc¶ŌÓ¦ĻµĘ×Ķ¼ÖŠµÄ4ŗÅøöĢå | |
| B£® | ŌÓ½»“ų2ĪŖÖĀ²”»łŅņÖĘ³ÉµÄĢ½Õė½ųŠŠ»łŅņ¼ģ²āŹ±ĖłŠĪ³ÉµÄŌÓ½»“ų | |
| C£® | 9ŗÅøöĢåÓėøĆŅÅ“«²”ÖĀ²”»łŅņŠÆ“ųÕß½į»é£¬ŗ¢×Ó»¼²”µÄøÅĀŹĪŖ$\frac{1}{8}$ | |
| D£® | 8ŗÅøöĢåµÄ»łŅņŠĶÓė3ŗÅøöĢåµÄ»łŅņŠĶĻąĶ¬µÄøÅĀŹĪŖ$\frac{2}{3}$ |
·ÖĪö ·ÖĪöĻµĘ×Ķ¼£ŗ1ŗÅŗĶ2ŗŶ¼Õż³££¬µ«ĖūĆĒµÄÅ®¶ł»¼²”£¬¼“£ŗĪŽÖŠÉśÓŠĪŖŅžŠŌ£¬ŅžŠŌæ“Å®²”£¬Å®²”ÄŠÕż·Ē°éŠŌ”±£¬ĖµĆ÷øĆ²”ĪŖ³£Č¾É«ĢåŅžŠŌŅÅ“«²”£®
·ÖĪö±ķøń£ŗ¶Ōl”«4ŗÅøöĢå·Ö±š½ųŠŠ»łŅņ¼ģ²ā£¬ÓÉÓŚ1”¢2ŗŶ¼ŹĒŌÓŗĻ×Ó£¬4ŗÅĪŖŅžŠŌ“æŗĻ×Ó£¬¶ų±ķøńÖŠa”¢bŗĶdĪŖŌÓŗĻ×Ó£¬cĪŖ“æŗĻ×Ó£¬Ōņ3ŗÅŅ²ĪŖŌÓŗĻ×Ó£¬cĪŖŅžŠŌ“æŗĻ×Ó£®
½ā“š ½ā£ŗA”¢øĆ²”ĪŖ³£Č¾É«ĢåŅžŠŌŅÅ“«²”£¬Ķ¼ÖŠ4ŗÅĪŖ»¼Õߣ¬ŹōÓŚŅžŠŌ“æŗĻ×Ó£¬¶ŌÓ¦ÓŚĻµĘ×Ķ¼ÖŠµÄc£¬AÕżČ·£»
B”¢Ķ¼2ÖŠcĪŖŅžŠŌ“æŗĻ×Ó£¬ŌņŌÓ½»“ų2ĪŖÖĀ²”»łŅņÖĘ³ÉµÄĢ½Õė½ųŠŠ»łŅņ¼ģ²āŹ±ĖłŠĪ³ÉµÄŌÓ½»“ų£¬BÕżČ·£»
C”¢9ŗŵĻłŅņŠĶ¼°øÅĀŹĪŖAA£Ø$\frac{1}{3}$£©»ņAa£Ø$\frac{2}{3}$£©£¬ĖūÓėŅ»øöŠÆ“ųÕߣØAa£©½į»é£¬ŗ¢×Ó»¼²”µÄøÅĀŹĪŖ$\frac{2}{3}$”Į$\frac{1}{4}$=$\frac{1}{6}$£¬C“ķĪó£»
D”¢Ļą¹Ų»łŅņÓĆA”¢a±ķŹ¾£¬øł¾ŻĶ¼2æÉÖŖ3ŗŵĻłŅņŠĶĪŖAa£¬øł¾ŻĶ¼1æÉÖŖ8ŗŵĻłŅņŠĶ¼°øÅĀŹĪŖAA£Ø$\frac{1}{3}$£©»ņAa£Ø$\frac{2}{3}$£©£¬Ņņ“ĖĮ½ÕßĻąĶ¬µÄøÅĀŹĪŖ$\frac{2}{3}$£¬DÕżČ·£®
¹ŹŃ”£ŗC£®
µćĘĄ ±¾Ģā½įŗĻĻµĘ×Ķ¼£¬æ¼²é³£¼ūµÄČĖĄąŅÅ“«²”£¬ŅŖĒóæ¼ÉśŹ¶¼Ē¼øÖÖ³£¼ūČĖĄąŅÅ“«²”µÄĄąŠĶ”¢ĢŲµć¼°ŹµĄż£¬ÄÜÕżČ··ÖĪöĢāĶ¼£¬²¢“ÓĶ¼ÖŠĢįȔӊŠ§ŠÅĻ¢“šĢā£¬ŹōÓŚæ¼øŁĄķ½ā²ć“ĪµÄ漲飮



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2015-2016ѧğŗž±±Ź”ĻåŃōŹŠøßŅ»ÉĻŌĀæ¼ÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠŠšŹöÄܶŌµ°°×ÖŹµÄ¹¦ÄܽųŠŠø߶ČøÅĄØµÄŹĒ£Ø £©
A£®Ļø°ūŗĶÉśĪļĢåµÄÖŲŅŖ½į¹¹ĪļÖŹ
B£®ŹÕĖõ”¢ŌĖŹä”¢ĆāŅßµČÉśĄķ»ī¶ÆµÄĪļÖŹ»ł“”
C£®ÉśĆü»ī¶ÆµÄÖ÷ŅŖ³Šµ£Õß
D£®µ÷½ŚĻø°ūŗĶÉśĪļĢåŠĀ³Ā“śŠ»µÄÖŲŅŖĪļÖŹ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2015-2016ѧğŗž±±Ź”¾£ÖŻŹŠø߶žÉĻŌĀæ¼ÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
Ö²ĪļµÄÖÖ×ÓĆČ·¢²¢³¤³ÉÓ×ĆēµÄ¹ż³ĢÖŠ£¬²»»į·¢ÉśµÄŹĒ£Ø £©
A£®ÖÖ×ÓµÄøÉÖŲ²»¶ĻŌö¼Ó
B£®¶ąÖÖ¼¤ĖŲ¹²Ķ¬µ÷½ŚĆČ·¢¹ż³Ģ
C£®ĶĮČĄ°å½įĻŌÖųÓ°ĻģĆČ·¢
D£®ø߶ū»łĢå²ĪÓėŠĀµÄĻø°ū±ŚŠĪ³É
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ø÷ÖÖÉśĪļµÄČ¾É«Ģå¾łæÉ·ÖĪŖ³£Č¾É«ĢåŗĶŠŌČ¾É«Ģå | |
| B£® | ČĖĄąµÄŠŌ±šČ”¾öÓŚ¾«×ӵĥąŠĶ£¬¹ŹŠŌ±š¾ö¶ØµÄŹ±¼äĪŖÄŠŠŌ¼õŹż·ÖĮŃ²śÉś¾«×ÓŹ± | |
| C£® | ČĖĄąĪ»ÓŚXŗĶYČ¾É«ĢåÉĻµÄ»łŅņŹōÓŚ°éŠŌŅÅ“« | |
| D£® | °éXČ¾É«ĢåĻŌŠŌŅÅ“«²”µÄÄŠŠŌ»¼Õ߶ąÓŚÅ®ŠŌ»¼Õß |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | XBXb£¬XBY | B£® | XbXb£¬XbY | C£® | XBXb£¬XbY | D£® | XbXb£¬XBY |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠÉśĪļ Ą“Ō“£ŗ2015-2016ѧğŗŚĮś½Ź”øßŅ»ÉĻµŚŅ»“ĪŌĀæ¼ÉśĪļŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠ¼øÖÖÉśĪļµÄĻø°ūÖŠ£¬ŹōÓŚŌŗĖĻø°ūµÄŹĒ£Ø £©

A£®¢Ł¢Ü B£®¢Ł¢Ś C£®¢Ū¢Ü D£®¢Ś¢Ü
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com