
�����������ŵ�ĹС˵�����У�������ѧ�ɲţ������Ĺ���У������һ�ε�Ĺ�ж��У����Ʋ�����һö�������룬��Ȼ̧�ţ����һ������ѽ���ҵ��찡����ͼ1Ϊ��ij���仡�ṹʾ��ͼ��A.E��M��NΪ���仡��λ�㣬DΪ���뼡ϸ����ͷ��λ����һ����B.C���Ƶ�ͻ�������ͼ����
��1�����Ĺ�˴��һ������ѽ���ҵ��찡���йص��Ǵ���Ƥ���������е�_____����
��2������Aʩ��һǿ�̼�����ϸ��Ĥ������λ�仯��____________�����̼�ͼ1��M�㣬��A��____(�ܡ�����)�������ֱ仯����������ͼ��_____��ֻ�ܵ����˷ܵ��¡������ñ걾���ڽϸ�Ũ�ȵ�KCl��Һ�У��̼�A�㣬A��______���ᡢ���ᣩ������λ�仯����Ĺ�˷�����ijҩƷ����������ֹ�������������ú�ķֽ⣬��ˣ����ú�ͻ������Ԫ�ı仯��____________��
��3�������˿ڹ�������˷�ѿ�߸˾�����__________�����������������������������ҽ�����ĵ�Ĺ�˻�á����˷硱������Ϊ��ע�������˷翹����Ѫ����н���Ԥ�������ƣ�ͬʱ�������������˷��������������߹��̣�����ͼ��ʾ��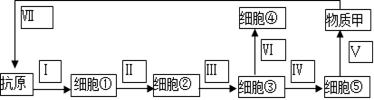
���У�����ʶ��ԭ��ϸ����ż�������[ ]_____________�������ʼϳɡ������йصĺ���֬��ϸ������__________��
��4���˸о�ʹ֮ǰ����̧�ŷ�Ӧ��������Ϊ__________���е��ڸû�ĵͼ����ࡣע��ʱ����Ĺ���ֽŲ�δ���أ���˵�����֡����ŷ��������___________�ĵ��ء�
��1��S�� ��2�������为 ���� B.C ���� �������˷ܻ����ƣ�3������ �ݽ�ϸ�� ���������߶����塢�����壨4������ ����Ƥ��
����������������˶���������������˵�˵�����ƾ�ͼ��֪��A�Ǵ�������ά������ά��Ϣʱ�ĵ�λ����Ϊ�����ڸ������ܵ��㹻ǿ�Ĵ̼�ʱ������������λ������Ϊ�⸺�������������˷���ͻ���������ǵ���ģ�����˷��ڷ��仡�еĴ����ǵ���ģ���ֻ�ܴӸ���������ЧӦ���������̼�ͼ1��M�㣬A�㲻���˷ܣ��������˷��Ե��ʺ������Ե��ʣ��������ֽ⣬��ᵼ��������ͻ����Ĥ�ϵ����������ϣ�ʹ��һ��Ԫ�������˷ܻ����ơ������˷�˾������������ͼ��֪��������Ϊ��Һ���ߣ���ϸ���٢ڢۢܢݷֱ�Ϊ����ϸ����Tϸ����Bϸ������ϸ���ͼ���Bϸ�������ʼ�Ϊ���塣���У�����ʶ��ԭ��ϸ���ǽ�ϸ�����뿹��ϳɡ������йصĺ���֬��ϸ���������������߶����塢�����塣��̧�ŷ�Ӧ�Ƿ��������䣬������������������ڼ��裻�ͼ������ܸ����ࣨ����Ƥ�㣩�Ŀ��ơ�
���㣺���⿼�����ߵ��ں��������֪ʶ�����ڿ��鿼����������ѧ֪ʶ��۵㣬ͨ���Ƚϡ��������ۺϵȷ�����ijЩ����ѧ������н��͡������������������жϻ�ó���ȷ�Ľ���������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��.�������°����ʣ��������зֽⱽ����������ϸ�����±���ɸѡ�ֽⱽ����������ϸ�����������䷽��
| KH2PO4 | 1.4 g |
| Na2HPO4 | 2.1 g |
| MgSO4��7H2O | 0.2 g |
| FeCl3 | 0.1 g |
| X | 1 g |
| ���� | �� |
| ��֬ | 15 g |
| �����������ܽ��������ˮ���ݵ�1 000 mL | |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
������ѧ�Ұ��������Ϊ��Ū�塰ת�����ӡ�Ϊ�����ʣ������˷���˫���ת������ʵ�顣���Ǵ�S�ͻ�ϸ������ȡ����DNA�������ʺͶ��ǵ����ʣ�Ȼ����ֱ��������R��ϸ��������������������Ҫ������ͼ��ʾ��˼�����ش��������⣺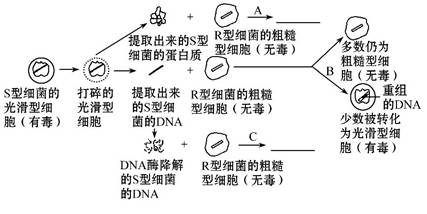
(1)��A��C���̵�Ԥ��������ͼ������ͼ�пո��ڡ�
(2)д��A��B��C��ʵ�������������⣺
A. _______________________________________________________________��
B. _______________________________________________________________��
C. _______________________________________________________________��
(3)��������ת�����Ӿ��� ��___________________________________
����ת�����ã��Ӷ�֤�� ____________________________________
_________________________________________________________________��
(4)ijͬѧͨ��ѧϰ����˫��������ں�����ת��ʵ����ʶ����R��ϸ��ת��ΪS��ϸ����ԭ����S��ϸ�����ڵ�ת�������ڷ������ã���ô������R��ϸ����ʹS��ϸ��ת��ΪR��ϸ������ģ�¸����˼�ȿ�ѧ�ҵ�ʵ����̽��������⣬��̽��R��ϸ���Ƿ���ת�����ӡ�
�ٽ�R��ϸ��ע��С�����ڣ�С��������
�ڽ�S��ϸ��ע��С�����ڣ�С��Ѫ֢������
�۽�����ɱ�����R��ϸ��ע��С�����ڣ�С��������
�ܽ�S��ϸ���ͼ���ɱ����R��ϸ����Ϻ�ע��С�����ڣ�С��Ѫ֢������
ʵ����ۣ�R��ϸ������û�С�ת�����ӡ���
��ͬѧ��ʵ���в���֮�����볢��˵�����ɡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
���ų���в���ཡ��������ɱ�֣�������������Ϊ��ϸ�����ȵ��ص������Խ������¡�ɽ�ηӿ���Ч���Ϳո�Ѫ�Ǻ��ȵ���Ũ�ȣ��Ԣ������������ԵĻ������ã���Ũ��Խ��Ч��Խ�ã�����Ϊ���������˴�����������ij�о����Դ���Ϊ����������֤��������������ṩ��ʵ��������þߣ����ʵ�鲽�裬��Ԥ��ʵ������
���ϣ�����״̬�����ء��Ա����ͬ����������50ֻ����ֲ�������Ƶĵ͡��С�������Ũ�ȵ�ɽ�ηӡ�ֲ���͡����������أ�STZ����ͨ���ϡ��������ϵȡ�
��ʾ��ֻ����������ϣ�ι��4�ܺ�ǻע��STZ�ɽ�������������ģ�ͣ�
��ҩ���������ָ��IJⶨ��������Ҫ��
���裺�ٷ��飺
��������ģ�ͽ�����
�۶���ʵ�飺
�ܽ����⣺
Ԥ��ʵ����������״ͼ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ͼ���������й����ں���Һ���ڲ��ֽṹʾ��ͼ���ش��������⣺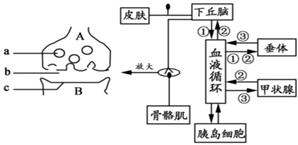
��1�������ܵ�����̼���ͼ�н�ͨ������ʹ�������ӵķ��仡��____��ͼʾ���آ٢ڢ۵����ϵ�����˼��ط��ڹ����о���_____�����ڷ�ʽ�����ص㡣
��2��Ѫ�Ǻ�������ʱ������Xͨ���ٽ�ϸ����_____ʹѪ�����ߡ�
��3��ϸ��A��ϸ��B֮����ӽ��IJ�λ��Ϊ____-��
��4��������������أ��������м���Ķ������ã�Ϊ���о�����ض�ͼ��ϸ��A��B֮���˷ܵĴ��ݹ����Ƿ���Ӱ�죬��ѧ�ҽ���������ʵ�飬������������±���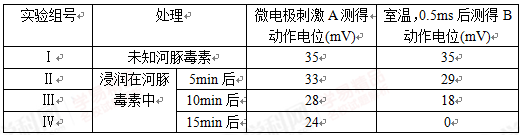
�ٵڢ��鴦��˵����Ԫ�˷�ʱ��Ĥ���Ĥ�ڵ�λ____��
�ڴӢ����жϣ�����ض����˷ܵĴ�����___���á�������ض���ͼ����ʶ����Ϣ���ӵ���������Ӱ�죬ͻ������Ԫ������λ�Ľ��Ϳ�����______ֱ������ġ�ͬʱ�����缫�̼�A��õĶ�����λ�ı仯�����Ʋ����ض�-____Ҳ���������ã������������_____��
�������ú���ص���������������ҩ�����____�����ţ���
a.����ҩ b.�ֲ����� c.��ʹ�� d.�����⾷�μ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
Ѫ��ƽ���������ڻ�����̬����Ҫ��ɲ��֣��ȵ�����ά��Ѫ��ƽ��������Ҫ���á���ͼ��ʾ�ȵ��ط��ڵĵ��ڹ��̼��ȵ������û�����������ش�
(1)Ѫ��ƽ��ĵ��ڷ�ʽ��_______��������λ��_________��
(2)��Ѫ������ʱ���ȵ�Bϸ�����������ǡ���_________����_______���źŷ��Ӵ̼�����������ӡ�ֱ�Ӳ����ȵ��ط��ڵ�Ĥ��ϸ������_________��
(3)�ȵ������õĻ����ǣ�һ����ͨ���ٽ�_______ �ٽ���֯ϸ����ȡ�����ǣ�ͬʱ��ͨ���ٽ����̢�_______���������ǵ����ã��Ӷ�����Ѫ��Ũ�ȡ�
(4)ij�ֿ���X�����ȵ�Bϸ��Ĥ�ϵ������������Ѫ���е�_______�½���Ѫ�������������ڻ�������ͬʱ����_______ ����
(5)�ٴ��ϣ��ڷ�����ҩ��ע���ȵ��ض�������II�������ߵ����ƣ��Ӷ������������ٵ�Ӱ�쿴���������Ʒ����У�����______________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
I���������°����ʣ��������зֽⱽ����������ϸ�����±���ɸѡ�ֽⱽ����������ϸ�����������䷽��
| KH2PO4 | 1.4g |
| Na2HPO4 | 2.1g |
| MgSO4��7H2O | 0.2g |
| FeCl3 | 0.1 g |
| X | 1g |
| ���� | �� |
| ��֬ | 15g |
| �����������ܽ��������ˮ���ݵ�100mL | |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ش������������������ص�����:
�Ŵ�ijֲ�ﳤ��һ�µĻƻ�������ȡ�ȳ����Σ���Ҷ�Ͳ�ѿ�����Ծ��ζ������¶Գ�������Լ3/4�������п��ľ��ν�û������ˮ�С�һ��ʱ��۲쵽��߾�����������������ͼ��ʾ���������ƻ��羥���е�������Ũ���Ǵٽ������ģ�����ˮ�к��߾��ڡ�������ϸ���е�������Ũ�ȶ��������ߡ�������������ص������ص������߾���������������һ�����Ʋ���ָ���������ֿ���ԭ��
�� �������ڲ�ϸ�������Ͽ졣��2�֣�
�� ����Ũ�ȵ������ظ��������ڲ�ϸ������������2�֣�
����ֲ������������ͶԻ�������Ӧ�����У����ص��ڷ�������Ҫ���ã����ڸ������ǻ�������һ����ʱ��Ϳռ��ϳ����Ա���Ľ��������������ĵ��ڻ���Ҫ����һЩ�����ڻ�������̬��Ҫ��������ڡ���Һ���ں����ߵ��ڹ�ͬ�������á�
�����ڵĻ�����ʽ�� ����Ԫ�����ڡ�����Ĵ̼�������˷ܣ���ͬһ����Ԫ�ڣ��˷��� ����ʽ�������ڲ�ͬ��Ԫ֮�䣬�˷�ͨ��ͻ����
�ķ�ʽ���ݡ�
�ڶ��D���� ���ڣ�ͨ�� ѭ�������͵�ȫ�����������ļ��ؿ�������Ӱ���ϸ�����������
������ϵͳ���� ���ܣ�������������Ҫͨ�� �������á�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
���������彡�������ŷdz���Ҫ�����ã�������ش��������⡣
��1�������ܷ��ڵ�֭���������ڵ����У���ʳ�ӽ���ʮ��ָ���������ҷ��ڵ�֭����ɸ÷���Ľṹ������____________��������нⶾ���ã�������ά��___ ���Ӷ���֤�����������������
��2���о���Ա���ָ�ϸ���ܽ���ȩת���ɼ���Ӷ������ȩ������Ϊ��֤��������ֹ��ܶ����������ʵ�飬ʵ�����������£����~����ʾ��û��ɵ�ʵ�鲽�裩��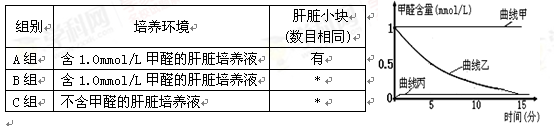
�ٱ��г�A���⣬������ø���С����� �顣
����ͼ������ ��C��ʵ��Ľ����˵����ϸ���ڴ�л�����л� ��
����ͼ���������� ��ʵ��Ľ������˵�� ��
��3���о�����һ��Ũ�ȵļ�ȩ���շ�Ⱦɫ����ѣ�Ϊ��һ����֤����Ľⶾ���ܣ��о���Աͬʱ����������ʵ�飺
��ȡ�������ľ��з��������������ܰ�ϸ����Һ3�ȷݣ����á�
�ڰ��ϱ���ʵ�������������15���Ӻ�ȡ����װ���е� ���ֱ���뱸�õ��ܰ�ϸ����Һ�м�������һ��ʱ�䡣�ٷֱ�ȡ�ܰ�ϸ��Ⱦɫ����Ƭ��Ȼ���������¹۲� �����Աȷ�����
��Ԥ��ʵ������������ ���еĸ�������Һ���ܰ�ϸ�������쳣��������������ܰ�ϸ��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com