
����������ض�ˮ�����������õ�ʾ��ͼ�����ʵ����֤���������õ������ԡ�
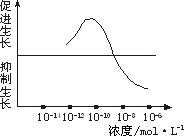
��1��ʵ����Ϻ��þߣ�ˮ���������ɣ���С��ͬ��װ�е�����ȫ����Һ�IJ���ƿ���ɣ���ͬŨ�ȵ���������Һ�����ߵȡ�
��2��ʵ�鲽�裺
��һ��������Ũ��Ϊ10��12 mol��L��10һ10 mol��L��10��8 mol��L��10��6 mol��L����������Һ����ȡ2 ml�ֱ����A��B��C��D�ĸ�ʢ����ͬӪ��Һ������ƿ�У�E����ƿ�г�����ͬ��Ӫ��Һ�⣬�ټ���________�������ա�
�ڶ�����ȡ����________��ˮ�����磬��������¼ˮ��������ij�ʼ���ȣ��ֱ������ڵ�A��B��C��D��E��ֻ����ƿ�С�
����������A��B��C��D��E����ˮ���������________�������£�����һ��ʱ�����________�������������ˮ�������������________�����μ�¼Ϊa��b��c��d��e��
��3��ʵ����ӦΪ��________��
 �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ������ʡʦ����2010-2011ѧ��߶���ѧ����ĩ������������ ���ͣ�071
��������������ض�ˮ�����������õ�ʾ��ͼ�����ʵ����֤���������õ������ԡ�
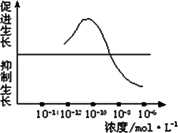
��1��ʵ����Ϻ��þߣ�ˮ���������ɣ���С��ͬ��װ�е�����ȫ����Һ�IJ���ƿ���ɣ���ͬŨ�ȵ���������Һ�����ߵȡ�
��2��ʵ�鲽�裺
��һ��������Ũ��Ϊ10��12 mol��L��10��10 mol��L��10��8 mol��L��10��6 mol��L����������Һ����ȡ2 ml�ֱ����A��B��C��D�ĸ�ʢ����ͬӪ��Һ������ƿ�У�E����ƿ�г�����ͬ��Ӫ��Һ�⣬�ټ���________�������ա�
�ڶ�����ȡ����________��ˮ�����磬��������¼ˮ��������ij�ʼ���ȣ��ֱ������ڵ�A��B��C��D��E��ֻ����ƿ�С�
����������A��B��C��D��E����ˮ���������________�������£�����һ��ʱ�����________�������������ˮ�������������________�����μ�¼Ϊa��b��c��d��e��
��3��ʵ���������ݴӴ�С������ӦΪ��________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��ͬ���� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
��09���Ƹ��е��У���6�֣���������������ض�ˮ�����������õ�ʾ��ͼ�����ʵ����֤���������õ������ԡ�
��1��ʵ����Ϻ��þߣ�ˮ���������ɣ���С��ͬ��װ�е�����ȫ����Һ�IJ���ƿ���ɣ���ͬŨ�ȵ���������Һ�����ߵȡ�
��2��ʵ�鲽�裺
��һ��������Ũ��Ϊ10��12mol��L��10һ10mol��L��10��8mol��L��10��6mol��L����������Һ����ȡ2ml�ֱ����A��B��C��D�ĸ�ʢ����ͬӪ��Һ������ƿ�У�E����ƿ�г�����ͬ��Ӫ��Һ�⣬�ټ���___________�������ա�
�ڶ�����ȡ����_____ _��ˮ�����磬��������¼ˮ��������ij�ʼ���ȣ��ֱ������ڵ�A��B��C��D��E��ֻ����ƿ�С�
����������A��B��C��D��E����ˮ��������� �������£�����һ��ʱ����� �������������ˮ������������� �����μ�¼Ϊa��b��c��d��e��
��3��ʵ����ӦΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
��09������һ������6�֣���������������ض�ˮ�����������õ�ʾ��ͼ�����ʵ����֤���������õ������ԡ�
��1��ʵ����Ϻ��þߣ�ˮ���������ɣ���С��ͬ��װ�е�����ȫ����Һ�IJ���ƿ���ɣ���ͬŨ�ȵ���������Һ�����ߵȡ�
 ��2��ʵ�鲽�裺
��2��ʵ�鲽�裺
��һ��������Ũ��Ϊ10-12mol��L��10-10mol��L��10-8mol��L��10-6mol��L����������Һ����ȡ2m1�ֱ����A��B��C��D�ĸ�ʢ����ͬӪ��Һ������ƿ�У�E����ƿ�г�����ͬ��Ӫ��Һ�⣬�ټ��� �������ա�
�ڶ�����ȡ���� ��ˮ�����磬��������¼ˮ��������ij�ʼ���ȣ��ֱ������ڵ�A��B��C��D��E��ֻ����ƿ�С�
����������A��B��C��D��E����ˮ��������� �������£�����һ��ʱ����� �������������ˮ������������� �����μ�¼Ϊa��b��c��d��e��
��3��ʵ����ӦΪ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com