
�������д��ڶ��������̵��������Ƕ����������еĵ�������Ҫ���塣ijʵ��С����ͼ�������з��������̵��������Ƴɾ���ʩ����������������������ʵ�����̴�������ͼ��ʾ����11�֣�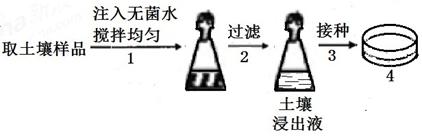
56��ͼ4�е��������ɷ����±���
| C6H12O6 | KH2PO4 | MgSO4 | NaCl | CaSO4 | ����ˮ | ��֬ | pH |
| 10g | 0.2g | 0.2g | 0.2g | 5g | 1000mL | 10g | 7.0 |
| ���ֵľ��� | һ�������� | ʵ�鴦������� |
| A | ������ | ����Ӫ����ף������� |
| B | ������ | ����Ӫ�����ң������� |
| A+B | ������ | ������Ӫ����ס��Ҿ������� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��2012���Ϻ����ɽ����и�����ѧ�����п���������ѧ�Ծ� ���ͣ��ۺ���
��13�֣��������д����Ŷ��������̵��������Ƕ������������еĵ�������Ҫ���塣ij������ȤС���ͬѧ��������з���������̵��������Ƶ��������ɷ�Ϊ������
��10g����������0.2g������þ0.2g���Ȼ���0.2g�������5g������ˮ1000mL����
���������м���1%����֬���Ƴɹ���������������ʵ��Ĺ�������ͼ�����������Т���
������Ʒ��������������Һ���ۡ��ܺ͢ݶ�������������ش�
1�����������Ĺ������֣���ʵ����������������� ��������̼ԴΪ ��
2��ʵ��ԭ����  ��
��
3���������������ľ����� ����䵪Դ������ ��
4���ڡ��۽��ֵķ����� �����ܡ��ݽ��ֵķ����� ����
5���ܡ��� ���ֲ�����Ŀ���� �����ֵ��ݵĹ�����Ϊ��ֹ��ȾӦ
���ֲ�����Ŀ���� �����ֵ��ݵĹ�����Ϊ��ֹ��ȾӦ
ע��IJ��������� ��
6����ʵ��ʹ�õ���ͨ�����������ڷ������������������Ӧ����___________�������ڼ���˾�������ͨ���������м���______________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2011-2012ѧ���Ϻ��и�����ѧ�����п���������ѧ�Ծ� ���ͣ��ۺ���
��13�֣��������д����Ŷ��������̵��������Ƕ������������еĵ�������Ҫ���塣ij������ȤС���ͬѧ��������з���������̵��������Ƶ��������ɷ�Ϊ������
��10g����������0.2g������þ0.2g���Ȼ���0.2g�������5g������ˮ1000mL����
���������м���1%����֬���Ƴɹ���������������ʵ��Ĺ�������ͼ�����������Т���
������Ʒ��������������Һ���ۡ��ܺ͢ݶ�������������ش�

1�����������Ĺ������֣���ʵ����������������� ��������̼ԴΪ ��
2��ʵ��ԭ���� ��
3���������������ľ����� ����䵪Դ������ ��
4���ڡ��۽��ֵķ����� �����ܡ��ݽ��ֵķ����� ����
5���ܡ��ݽ��ֲ�����Ŀ���� �����ֵ��ݵĹ�����Ϊ��ֹ��ȾӦ
ע��IJ��������� ��
6����ʵ��ʹ�õ���ͨ�����������ڷ������������������Ӧ����___________�������ڼ���˾�������ͨ���������м���______________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ���Ϻ���¬��������4��ģ�⿼�������Ծ� ���ͣ��ۺ���
�������д��ڶ��������̵��������Ƕ����������еĵ�������Ҫ���塣ijʵ��С����ͼ�������з��������̵��������Ƴɾ���ʩ����������������������ʵ�����̴�������ͼ��ʾ����11�֣�
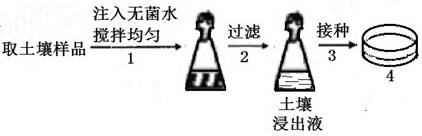
56��ͼ4�е��������ɷ����±���
|
C6H12O6 |
KH2PO4 |
MgSO4 |
NaCl |
CaSO4 |
����ˮ |
��֬ |
pH |
|
10g |
0.2g |
0.2g |
0.2g |
5g |
1000mL |
10g |
7.0 |
�����������ص���______________��ԭ����____________________________
________________________________________________________________��
57���ӹ����Ͽ���������������__________������������̬�Ͽ��������������ڹ�����������������__________________________________��
58����Ϊ��֬�г����е������ʹ��ǰ��Ҫ������ˮ��ˮ��ϴ���죬����________________���ݣ�ϴ�Ӽ��Σ��Գ�ȥ���еĵ���
59��ʵ�鲽��3�ڳ���̨�ϣ������ֻ���____________�������ȴ����պȡ����ϡ��Һ�õ�ӷ����������������棬Ҳ�ɲ���_______�����֡����ֺ���뽫������_______________��������������ˮ���䡣
60�� ʵ�������ʱ�䲻�˹���������____________________________________
____________________________��
61.С���Ա�÷����ߴ�������õ��Ĺ̵����������ͻ����A��B��Ȼ���A��B����ʵ�飬������±���
|
���ֵľ��� |
һ�������� |
ʵ�鴦������� |
|
A |
������ |
����Ӫ����ף������� |
|
B |
������ |
����Ӫ�����ң������� |
|
A+B |
������ |
������Ӫ����ס��Ҿ������� |
(1)ͻ����A������һ����������������ֱ��ԭ����_____________________��
(2)��ͻ����A��B�����һ�������һ����������ȴ��������������ô���
��ԭ����
��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��Ķ�����
�������д��ڶ��������̵��������Ƕ����������еĵ�������Ҫ���塣ijʵ��С����ͼ�������з��������̵��������Ƴɾ���ʩ����������������������ʵ�����̴�������ͼ��ʾ��

56��ͼ4�е��������ɷ����±���
| C6H12O6 | KH2PO4 | MgSO4 | NaCl | CaSO4 | ����ˮ | ��֬ | pH |
| 10g | 0.2g | 0.2g | 0.2g | 5g | 1000mL | 10g | 7.0 |
�����������ص���______________��ԭ����____________________________
________________________________________________________________��
57���ӹ����Ͽ���������������__________������������̬�Ͽ��������������ڹ�����������������__________________________________��
58����Ϊ��֬�г����е������ʹ��ǰ��Ҫ������ˮ��ˮ��ϴ���죬����________________���ݣ�ϴ�Ӽ��Σ��Գ�ȥ���еĵ���
59��ʵ�鲽��3�ڳ���̨�ϣ������ֻ���____________�������ȴ����պȡ����ϡ��Һ�õ�ӷ����������������棬Ҳ�ɲ���_______�����֡����ֺ���뽫������_______________��������������ˮ���䡣
60�� ʵ�������ʱ�䲻�˹���������____________________________________
____________________________��
61.С���Ա�÷����ߴ�������õ��Ĺ̵����������ͻ����A��B��Ȼ���A��B����ʵ�飬������±���
| ���ֵľ��� | һ�������� | ʵ�鴦������� |
| A | ������ | ����Ӫ����ף������� |
| B | ������ | ����Ӫ�����ң������� |
| A+B | ������ | ������Ӫ����ס��Ҿ������� |
(1)ͻ����A������һ����������������ֱ��ԭ����_____________________��
(2)��ͻ����A��B�����һ�������һ����������ȴ��������������ô���
��ԭ����
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com