
�澳��ָ��ֲ������˺��Ļ���������������պɺ��ȣ�������������ݻش����⡣
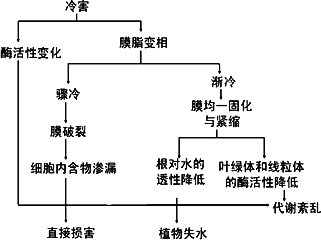
��ũ���������ֱ���ܵ��亦�Ͷ�����Ӱ�졣ũ�����亦����Ҫ�����������ڱ������ϵ���ʱ������Ĥ��֬����Һ��ת��Ϊ���࣬��Ĥ֬���࣬������Ĥ���ϵ�øʧ�ֲ���亦����Ҫ���Ƽ���ͼ��ʾ�����⣬�������µ��¶�ֲ���Σ���ж�����
������Ҫ�ǵ����γɵı�����ϸ�����˺���ϸ���ڽ�����ϸ��Ĥ��ϸ������������ϸ�������ƻ����ã��Ӷ���ֲ������������ˡ���������е���Ϣ�ش��������⣺
1��ֲ�������亦���ж��ֱ��֣����¸��ֱ����У���Щ�����亦�ı���
A�������
B������й©
C��������ʼ���
D��������ǿ
2��ϲ��ֲ�ﴦ�����ϵ��»��ܵ��˺��������������������Ϊ�亦��ԭ�������ǵ����˺�������Ĥ����ʱ�ܺ�ֲ��Ĥ����________��������________��
3��Ϊʲô�ڵ��������£����˸�����ʩ�ʣ���˵���������ɣ�________��________��
��ֲ����澳�ĵֿ������Dz�һ���ģ����Ŵ����غ�������ص�˫��Ӱ�졣ֲ����澳�Ŀ���ǿ����ֲ��ĵ絼�ʵͣ���֮���絼�ʾߡ��絼�ʿ�����һ���ļ����ֶβ��������������Ϊ�����ᣨһ��ֲ�D�أ��������ֲ����澳�ĵֿ�������ʹ�����в������һ��ʵ�飬̽���������ֲ����Ե�Ӱ�졣
������Ʒ������������ƿ��״̬������һ�µĻƹ�����10�ꡢ����Ũ�ȵ������ᡢ�ⶨ�絼�ʵ�װ�á�0.25 mol��L��NaCl��Һ������ˮ��
4��ʵ���е��Ա�����________��
5��ʵ����裺________��
6��ʵ�鲽�裺
��һ����________��
�ڶ�������ס�������ƹ�������������зֱ���������������0.25 mol��L��NaCl��Һ��
��������________��
���IJ���________��
7��Ԥ�ڽ�������ۣ�________
|
����1��d ����2�����ӡ��½� ����3������ʹĤ��ø�Ļ����½������������ӵ��������������½������������ҺŨ�����ߣ�������ˮ�����½�������ʧˮ��2�֣� ����4�������� ����5��������������ֲ����澳�ĵֿ��� ����6����һ����������������ƿ��״̬������һ�µĻƹ�����10��ƽ���ֳɼס������� �������������������ʩ��������������Һ����������ʩ������ˮ�� �������IJ����ֱ�ⶨ�ס�������ĵ絼�ʡ� ����7��Ԥ�ڽ������ۣ���ÿ��1�֣���3�֣� ��������ס�������ĵ絼����ȣ���˵������������ֲ����澳�ĵֿ����� �����������絼�ʴ�������ĵ絼�ʣ���˵����������Խ���ֲ����澳�ĵֿ����� �����������絼��С������ĵ絼�ʣ���˵��������������ֲ����澳�ĵֿ����� |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��2011���Ϻ����ɽ�������5��ģ����������Ծ� ���ͣ��ۺ���
�澳��ָ��ֲ������˺��Ļ���������������պɺ��ȣ�������������ݻش����⡣
��.ũ���������ֱ���ܵ��亦�Ͷ�����Ӱ�졣ũ�����亦����Ҫ�����������ڱ������ϵ���ʱ������Ĥ��֬����Һ��ת��Ϊ���࣬��Ĥ֬���࣬������Ĥ���ϵ�øʧ�ֲ���亦����Ҫ���Ƽ���ͼ��ʾ�����⣬�������µ��¶�ֲ���Σ���ж�����
������Ҫ�ǵ����γɵı�����ϸ�����˺���ϸ���ڽ�����ϸ��Ĥ��ϸ������������ϸ�������ƻ����ã��Ӷ���ֲ������������ˡ���������е���Ϣ�ش��������⣺
70��ֲ�������亦���ж��ֱ��֣����¸��ֱ����У���Щ�����亦�ı��֣� ��
| A����л���� | B������й© | C��������ʼ��� | D��������ǿ |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010�콭����ͨͨ������У���������Ծ��������� ���ͣ��ۺ���
��ĩֲ���ҶƬ˥��ʱ�� ��Ҷ��������ʼ�γ���㣨��ͼ����ʾ���������ֲ���������䡣��㲿λϸ����ص��������ͼ����ʾ�����ͼ�ش�����
��Ҷ��������ʼ�γ���㣨��ͼ����ʾ���������ֲ���������䡣��㲿λϸ����ص��������ͼ����ʾ�����ͼ�ش����� ���⣺
���⣺ ��1������ͼ��֪��Ҷ���������IJ�������ߣߣߣߣ�ֲ�D�أ������й�ϵ��
��1������ͼ��֪��Ҷ���������IJ�������ߣߣߣߣ�ֲ�D�أ������й�ϵ��
�� 2��A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬��
2��A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬�� �ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ��ϸ��Ĥ�ģߣߣߣ����ã����ڵ�ϸ���⡣
�ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ��ϸ��Ĥ�ģߣߣߣ����ã����ڵ�ϸ���⡣
��3���ù��̷��ڵ�ϸ����֮������ʣ�������γ�����������ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4����һ�����о�������Ҷ�����������γɣ�������������������صIJ��룬��˵���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��5���澳��ָ��ֲ������˺��Ļ���������������պɺ��ȡ�ֲ����澳�ĵֿ������Dz�һ���ģ����Ŵ����غ�������ص�˫��Ӱ�졣ֲ����澳�Ŀ���ǿ����ֲ��ĵ絼�ʵͣ���֮���絼�ʾߡ��絼�ʿ�����һ���ļ� ���ֶβ��������������Ϊ������������ֲ����澳�ĵֿ�������ʹ�����в������һ��ʵ�飬��̽���������ֲ����Ե�Ӱ�졣������Ʒ������������ƿ��״̬������һ�µĻƹ�����10�ꡢ����Ũ�ȵ������ᡢ�ⶨ�絼�ʵ�װ�á�0.25mol��L NaCl��Һ������ˮ��
���ֶβ��������������Ϊ������������ֲ����澳�ĵֿ�������ʹ�����в������һ��ʵ�飬��̽���������ֲ����Ե�Ӱ�졣������Ʒ������������ƿ��״̬������һ�µĻƹ�����10�ꡢ����Ũ�ȵ������ᡢ�ⶨ�絼�ʵ�װ�á�0.25mol��L NaCl��Һ������ˮ��
��ʵ�鲽�裺
��һ������10��ƹ�����ƽ���ֳɼס������飻 �ڶ������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ڶ������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
���������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
���IJ����ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Ԥ�ڽ�������ۣ��ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ���ӱ�ʡ2010�����һģģ�⣨�����������ﲿ�� ���ͣ��ۺ���
��13�֣��澳��ָ��ֲ������˺��Ļ���������������պɺ��ȡ�ֲ����澳�ĵֿ������Dz�һ���ģ����Ŵ����غ�������ص�˫��Ӱ�졣ֲ����澳�Ŀ���ǿ����ֲ��ĵ絼�ʵͣ���֮���絼�ʾߡ��絼�ʿ�����һ���ļ����ֶβ��������������Ϊ������������ֲ����澳�ĵֿ�������ʹ�����в�����ơ���ʵ�飬��̽���������ֲ����Ե�Ӱ�졣
������Ʒ������������ƿ��״̬�����ҡ��µĻƹ�����10�ꡢ����Ũ�ȵ������ᡢ�ⶨ�絼�ʵ�װ�á���ȫ������Һ������ˮ��
��1��ʵ���е��Ա�����__________________��
��2��ʵ����裺___________________________________��
��3��ʵ�鲽�裺
�ڡ�������10��ƹ�����ƽ���ֳɼס�������
�ڶ�����______________________________________��
��������______________________________________��
���IJ���______________________________________��
��4��Ԥ�ڽ�������ۣ�______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ���Ϻ����ɽ�������5��ģ����������Ծ� ���ͣ��ۺ���
�澳��ָ��ֲ������˺��Ļ���������������պɺ��ȣ�������������ݻش����⡣
��.ũ���������ֱ���ܵ��亦�Ͷ�����Ӱ�졣ũ�����亦����Ҫ�����������ڱ������ϵ���ʱ������Ĥ��֬����Һ��ת��Ϊ���࣬��Ĥ֬���࣬������Ĥ���ϵ�øʧ�ֲ���亦����Ҫ���Ƽ���ͼ��ʾ�����⣬�������µ��¶�ֲ���Σ���ж�����

������Ҫ�ǵ����γɵı�����ϸ�����˺���ϸ���ڽ�����ϸ��Ĥ��ϸ������������ϸ�������ƻ����ã��Ӷ���ֲ������������ˡ���������е���Ϣ�ش��������⣺
70��ֲ�������亦���ж��ֱ��֣����¸��ֱ����У���Щ�����亦�ı��֣� ��
A.��л���� B.����й© C.������ʼ��� D.������ǿ
71��ϲ��ֲ�ﴦ�����ϵ��»��ܵ��˺��������������������Ϊ�亦��ԭ�������ǵ����˺�������Ĥ����ʱ�ܺ�ֲ��Ĥ����____________��������_____________��
72��Ϊʲô�ڵ��������£����˸�����ʩ�ʣ���˵���������ɣ� ��
��
��.ֲ����澳�ĵֿ������Dz�һ���ģ����Ŵ����غ�������ص�˫��Ӱ�졣ֲ����澳�Ŀ���ǿ����ֲ��ĵ絼�ʵͣ���֮���絼�ʾߡ��絼�ʿ�����һ���ļ����ֶβ��������������Ϊ�����ᣨһ��ֲ�D�أ��������ֲ����澳�ĵֿ�������ʹ�����в������һ��ʵ�飬̽���������ֲ����Ե�Ӱ�졣
������Ʒ������������ƿ��״̬������һ�µĻƹ�����10�ꡢ����Ũ�ȵ������ᡢ�ⶨ�絼�ʵ�װ�á�0��25mol/L NaCl��Һ������ˮ��
73��ʵ���е��Ա�����______________________________��
74��ʵ����裺___________________________________________________��
75��ʵ�鲽�裺
��һ����__________________________________________________________________��
�ڶ�������ס�������ƹ�������������зֱ���������������0.25mol/L NaCl��Һ��
��������__________________________________________________________________��
���IJ���__________________________________________________________________��
76��Ԥ�ڽ�������ۣ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010��ɽ��ʡ�߶���ѧ�����п��������Ծ� ���ͣ��ۺ���
�澳��ָ��ֲ������˺��Ļ����������ա������ɺ��ȡ�ֲ����澳�Ŀ���ǿ����ֲ��ĵ絼�ʵͣ���֮���絼�ʾߡ��絼�ʿ�����һ���ļ����ֶβ��������������Ϊ������������ֲ����澳�ĵֿ�������ʹ�����в������һ��ʵ�飬��̽���������ֲ����ԣ������գ���Ӱ�졣
������Ʒ������������ƿ��״̬������һ�µĻƹ�����10�ꡢ����Ũ�ȵ������ᡢ�ⶨ�絼�ʵ�װ�á�0.25mol��L NaCl��Һ������ˮ����ע���������������ֲ��ķ�ʽ����ֲ�
��ʵ�鲽�裺��һ������10��ƹ�����ƽ���ֳɼס������飻
�ڶ����� ��
�������� ��
���IJ��� ��
��Ԥ�ڽ�������ۣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com