
ijũ���ڳ��������ͬʱ����ˮ����ֲ���IJˡ����ס�ˮ�۵��߲ˣ��Ա��ø���ľ���Ч�档
(1)�ó�����̬ϵͳ�Ľṹ����________��________��
(2)�����������õ�������ʳ�ú�����ȫ�������գ���й��ˮ�к���Ҫ��A________(����̬ϵͳ����ɳɷ�)�ֽ⣻��A������ֳ����A���������ˮ�е�________�����������������
(3)������Σ���߲˵ĺ��棬Ϊ�˷�ֹ�߲˵ļ�������ֳ����������ʩ����һ��������ư�棬��������з��Σ������ͼ��ʾ������������Ⱥ�ܶȵķ�����________����ͼ�����������Ⱥ�ܶ��½���Ϊ���Ե�ʱ�����________������Ⱥ�����ĽǶȷ���������8��9�·�������Ⱥ�ܶȺܵ͵���Ҫԭ����______________________________________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ÿ��2�֣���12�֣��±���H2O2�ڲ�ͬ�����µķֽ⡣�ݱ��ش�
| �Թܺ� | ���� | ���� |
| 1 | 2 mL H2O2��2������ˮ | �������ݺ��� |
| 2 | 2 mL H2O2��2��FeCl3 | �������ݽ϶� |
| 3 | 2 mL H2O2��2�θ�����ĥҺ | �������ݺܶ� |
| 4 | 2 mL H2O2��2��(���)������ĥҺ | ����ͬ1 |
| 5 | 2 mL H2O2��2�θ�����ĥҺ��2��5%HCl | ����ͬ1 |
| 6 | 2 mL H2O2��2�θ�����ĥҺ��2��5%NaOH | ����ͬ1 |
| 7 | | ����ͬ3 |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ȥ���Ե�����������ܣ��Ƽ��ܣ�Ϊ���ϣ����з���ʵ�顣��ش�����йص����⣺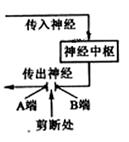
��1������̼��������֫��ֺ�����ɹ۲쵽�ú�֫������������÷����ĸ�����λ�����ֺ֫���� �У�������λ�� �С�
��2���������ǴӸ��������ܴ̼���ʼ��ЧӦ��������Ӧ��������һ���������� �������ġ�
��3������֧�伹�����֫�Ĵ���������ͼ���������̼�A�� ���ܡ����ܣ��������֫��������̼�B�� ���ܡ����ܣ��������֫����������̼����ϴ���ijһ�˳�����������û ���ܡ����ܣ���Ϊ��������Ҫԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ͼ1��ij��̬ϵͳ����������ͼ�⣬ͼ����ֵ�ĵ�λΪkcal(m2��a)��ͼ2��ij���������õ��ϵ�ֲ��á�������ӥ���ɵ�ʳ������ij��ʱ�ڣ����ؾ��������ɱ�������µ�������Ⱥ��������������߸˾����������ͨ������ʹ�˸�Ⱦ����ش����⣺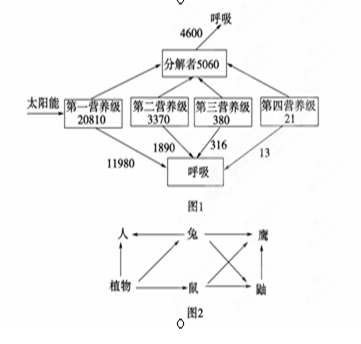
(1)ͼ1�еڶ�Ӫ�����������������Ӫ�����Ĵ���Ч��ԼΪ________%(����һλС��)��
(2)ͼ1���ݷ�ӳ������̬ϵͳ������Ⱥ��û�����浽����ȶ��ĽΣ��ж���������������________(����ڡ��������ڡ���С�ڡ�)���������
(3)ͼ2��ʾʳ�����У�ֲ���е�������ͨ��________��ʳ�������ݸ�ӥ��ӥ������Ӫ������________���ú��˵��ּ��ϵ�� �����߸˾����˵��ּ��ϵ��________��
(4)ͨ������£���������Ⱥ������Ӱ��____________(����ڡ�����С�ڡ����ڡ�)�����������ϵ����������Ⱥ������Ӱ�죻������ɱ���ᵼ�������ڶ���________(���ǿ���������䡱������)��
(5)�����ڸõ��ϵ�Ģ�����ڴ��������________(��ܡ����ܡ�)���ݸ�ֲ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��ͼΪ�´���ԭʳ��������ͼ��
��ش�
(1)ӥ����������Ⱥ֮��Ĺ�ϵΪ____________________________��
(2)��ԭ��̬ϵͳ��ɭ����̬ϵͳ��Ȼָ����ȶ��Խ�_____��
(3)��ͼΪ�ò�ԭ��̬ϵͳ����������ʾ��ͼ
�����������ص���_____ ____��ͼ�е�A��B�ֱ��ʾ�ĺ�����_________��_________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��7�֣���ͼһˮ��ϸ���ڷ�������������ʾ��ͼ��1��10��ʾ�������̣�A��D��ʾ��ѧ���ʣ�ͼ����ʾ������PEPCø������ˮ����õĹ���ǿ�ȶ�ת����ˮ����ԭ��ˮ���Ĺ�����ʵ�Ӱ�������������
��1��ͼһ�д���������ð���Ӧ���� ��A��B���������ʷֱ���__________________����ͼ��ʾ��л�����У����Է����ڸߵȶ���ϸ���ڵ�����������________________��
��2��ͨ��һ��������PEPCø������ˮ��ϸ����Ҫͨ��________________�����γ�ˮ�����塣���������㣬����ǿ��Ϊ1��102��mol��m-2��s-1ʱ��ͼһ�пɽ��е�����������______________��
��3������ͼ���������ڹ���ǿ��Ϊ4��12����102��mol��m-2��s-1��ʱ��ת����ˮ���ĺϳ�O2��ԭ��ˮ���ϳɵ�O2�ı�ֵ�ֱ�Ϊ______________________�����ת����ˮ�����ʺ�������_________________�����С�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��������(SW)��ӻҿ������з������������������Ϊ�ǡ�δ������������ҩ�����������ص�ʵ���о����̼������
�ٽ�������С��ΰ�Hepal��6ϸ����Һ���ֱ���������ɸ����е�������Һ������ƿ�У�
�ڽ�����ƿ����37 �桢5% CO2������������24 h�����á�ȥ������Һ��
�۷ֱ�������������ͬŨ��SW������Һ����37 �桢5% CO2�������м���������
�ֱܷ���24 h��48 h��72 hʱ��ȡ����Һ���۲������õ���ͬŨ��SW��ϸ�������Ӱ��������ͼ��ʾ��
��ش��������⡣
(1)��������5% CO2��������______________________________________��
(2)���ڼ��ڵĸΰ�Hepal��6ϸ���������ˮƽ����Ҫ�仯��________________________________________________________________��
(3)���������Ҫ���ö����飬������Ĵ�������ӦΪ________���������ÿ��ʵ��������5������ƿͬʱ����������������ͳ��ƽ��ֵ������Ϊ��________________��ʹʵ������ȷ��
(4)����ͼ�����߿�֪��SW�Ըΰ�Hepal��6ϸ������Ч�����ص��Ǣ�______________________________________________________________��
��__________________________________________________________��
(5)������48 h������Һ���ģ�ȥ������Һ��һϵ�еĴ������������õ�����48 hϸ����Ŀ����������Bax��Bcl��2�ı����������ʾ��
| SW | ��ϸ����Ŀ | ����ϸ����Ŀ | Bax���� | Bcl��2���� |
| 2/(��g��mL��1) | �� | ������ | ���������� | �� |
| 1/(��g��mL��1) | ���� | ������ | ������ | ���� |
| 0.5/(��g��mL��1) | ������ | ���� | ���� | ������ |
| 0/(��g��mL��1) | �������� | �� | �� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��¡���ɹ��ʽϵͣ���������̥ϸ�����쳣�����йء�Bcl��2������ϸ���������ƻ�����PCR�������Լ��û���ת¼ˮƽ�������˽�û����벻ͬ��̥ʱ��ϸ�������Ĺ�ϵ����¡�����������û���ת¼ˮƽ���������ͼ��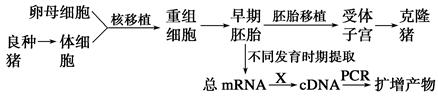
��ش�
(1)ͼ������ϸ����ϸ��������________ϸ����������̥���������ӹ��������������ɣ��ߡ����ߺ�______�����շ���Ϊ��¡����
(2)��PCR�����пɼ���cDNA��Bcl��2 cDNA�ķ�����������������mRNA��Bcl��2mRNA�ķ��������Ӷ���ӳ��Bcl��2�����ת¼ˮƽ��
��ͼ��X��ʾ______���̡�
�ڴӻ��������ݿ��в�ѯBcl��2mRNA�ĺ��������У��Ա������һ������ƺϳ�________����PCR������PCR���̵�һ��ѭ����ģ����________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��ۺ���
��5�֣�ijͬѧ�Բ��˵���ҶΪ���ϣ��Ʊ�������Ҷ��������Һ�����Ʊ���Ҷ��������Һ����Ϊ���飬һ��Ϊʵ���飬��һ������飬������ʵ�����м������������ᣨPi�����������¶Ⱥ��յ������£���14C��ǵ�14CO2������й�����ã�Ȼ���ټ������ԣ��������ʵ����14C��ǻ������������CH2O��/C3��ֵ���ȶ��������Ըߡ�
��1����ʵ���Ŀ����___ ��
��2��ʵ����14C��ǻ������������CH2O��/C3��ֵ���ȶ��������Ըߵ�ԭ����
��
��3���Բ��˵���ҶΪ�����ø߱����۲�Ҷ���壬��ƬʱӦȡ����Ҷ �����ز�Ƭ��ˮ���У�Ȼ�� ����װƬ���������½��й۲졣
��4������������Ҷ������Ҫ�õ��ķ����� ���ˡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com