
��������NH+4��PO3-4��K+��Ca2+��ͬ����500mLˮ�У��ٷ���һЩ���ʵ�ˮ�����⣬��Сʱ��ⶨ���Һ�������������Ӻ�ˮ�ĺ����仯���£�
| �� Ŀ | H2O | NH+4 | K+ | Ca2+ | PO3-4 |
| ������ | 0% | 83% | 72% | 97% | 84% |
C
��������������ɱ������ݿ��Կ�������Ũ�ȱ仯�ϴ�ˮ�ֺ���ǰ����Բ��䣬������Ԫ��ֻ��������״̬���ܱ�ˮ���գ���ˣ�ֲ������ˮ�ֺͿ���Ԫ����������Զ����Ĺ��̡�4������ԭ��Ũ����ͬ����һ���Ũ���в��죬˵��������Щ���Ӷ������գ��ҶԿ���Ԫ�ص����վ���ѡ���ԣ�һ���Ca2+Ũ����ͣ������յ���࣬˵��ˮ����ϸ��Ĥ������Ca2+������������������ӵ�����ࡣ��ѡC
���㣺���⿼��ֲ�����ˮ�ֺͿ���Ԫ�����յ����֪ʶ��
�������������ڿ���ѧ����������������������е��Ѷ��⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��2015�����ɹŻ��ֹ����и�һ12���¿������Ծ��������棩 ���ͣ�ѡ����
��������NH+4��PO3-4��K+��Ca2+��ͬ����500mLˮ�У��ٷ���һЩ���ʵ�ˮ�����⣬��Сʱ��ⶨ���Һ�������������Ӻ�ˮ�ĺ����仯���£�
|
�� Ŀ |
H2O |
NH+4 |
K+ |
Ca2+ |
PO3-4 |
|
������ |
0% |
83% |
72% |
97% |
84% |
���з����������ȷ����
�����Ӵ������ٶ�ˮȴû�м��٣�˵������ˮ�ֵ����պͶԿ������ӵ�������������Զ����Ĺ���
���������Ӷ����٣�˵��������Щ���Ӷ�������
�۸����������ӵ��������в��죬˵���������ӵ����վ���ѡ����
��Ca2+��K+���Լ��٣�˵��ˮ����ϸ��Ĥ������Ca2+�����������K+�������
A���٢ڢ� B���٢ۢ� C���ڢۢ� D������ȷ
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ�꽭��ʡ������һ���¿��������� ���ͣ�ѡ����
��������NH+4��PO3-4��K+��Ca2+��ͬ����500mLˮ�У��ٷ���һЩ���ʵ�ˮ�����⣬��Сʱ��ⶨ���Һ�������������Ӻ�ˮ�ĺ����仯���£�
|
�� Ŀ |
H2O |
NH |
K+ |
Ca2+ |
PO |
|
������ |
0% |
83% |
72% |
97% |
84% |
���з����������ȷ���� �� ��
�����Ӵ������ٶ�ˮȴû�м��٣�˵������ˮ�ֵ����պͶԿ������ӵ�������������Զ����Ĺ���
���������Ӷ����٣�˵��������Щ���Ӷ�������
�۸����������ӵ��������в��죬˵���������ӵ����վ���ѡ����
��Ca2+��K+���Լ��٣�˵��ˮ����ϸ��Ĥ������Ca2+�����������K+�������
A���٢ڡ�������B���٢ۡ�������C���ڢۡ�������D������ȷ
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2011�콭��ʡ�ϲ�һ�и�����һ���¿��������� ���ͣ���ѡ��
��������NH+4��PO3-4��K+��Ca2+��ͬ����500mLˮ�У��ٷ���һЩ���ʵ�ˮ�����⣬��Сʱ��ⶨ���Һ�������������Ӻ�ˮ�ĺ����仯���£�
| �� Ŀ | H2O | NH | K+ | Ca2+ | PO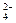 |
| ������ | 0% | 83% | 72% | 97% | 84% |
| A���٢� | B���٢� | C���ڢ� | D������ȷ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com