
��ӦCH3OH ( l )+ NH3( g ) = CH3NH2( g ) + H2O ( g )��ij�¶��Է����ҽ��У�����Ӧ����H��= 17kJ/mol������H��T��S��= 17kJ/mol�����ڸ÷�Ӧ���й�ϵ��ȷ���ǣ� ��
A. ��H ��0����H�CT��S��0 B. ��H ��0����H�CT��S��0
C. ��H ��0����H�CT��S��0 D. ��H ��0����H�CT��S��0

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
����������CO2������ϳɼ״�������о��ܵ�Խ��Խ��Ĺ�ע���÷����ȿɽ��CO2�������������⣬�ֿɿ��������״�����;�����������õ�Ӧ��ǰ������֪4.4 g CO2������H2������������CH3OH�����ˮ����ʱ�ų�4.95 kJ��������
(1)�÷�Ӧ���Ȼ�ѧ����ʽΪ��__________________________________________.
(2)��270�桢8 MPa���ʵ������������£�CO2��ת���ʴﵽ22%����4.48 m3(���ۺ�Ϊ��״��)��CO2�ںϳ�CH3OH����������ܷų�����________ kJ.
(3)����֪H2O(g)===H2O(l)����H����44 kJ/mol����CO2������H2���巴Ӧ����CH3OH����16g��Һ̬ˮʱ�ų�����Ϊ kJ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ�긣��ʡ������ѧ�ڵ�����ģ�⿼�ԣ����ۣ����ﲿ�� ���ͣ�ѡ����
��֪ij�¶������ܱ������м���CH3OH���������¿��淴Ӧ��
2CH3OH��g��==CH3OCH3��g��+H2O��g������Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�ȣ�mol��L-1�����£������й�˵������ȷ���ǣ� ��
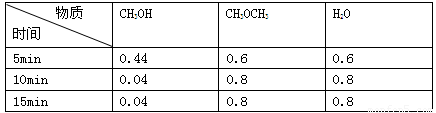
A��10minʱv��=v��
B��5minʱ�÷�Ӧ����v(CH3OH)=0.088mol��L-1��min-1
C�����¶��´˷�Ӧ��ƽ�ⳣ��k=400
D��ƽ��������������䣬���£�c(CH3OH)=0.06mol/L���÷�Ӧ��H<0
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2011�츣��ʡ�������и�����ѧ�ڵ�����ģ�⿼�ԣ����ۣ����ﲿ�� ���ͣ���ѡ��
��֪ij�¶������ܱ������м���CH3OH���������¿��淴Ӧ��
2CH3OH��g��==CH3OCH3��g��+H2O��g������Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�ȣ�mol��L-1�����£������й�˵������ȷ���ǣ� ��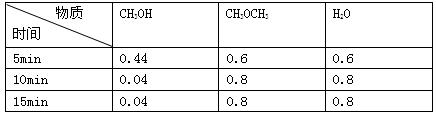
| A��10minʱv��=v�� |
| B��5minʱ�÷�Ӧ����v(CH3OH)=0.088mol��L-1��min-1 |
| C�����¶��´˷�Ӧ��ƽ�ⳣ��k=400 |
| D��ƽ��������������䣬���£�c(CH3OH)=0.06mol/L���÷�Ӧ��H<0 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com