
�����й���������ϣ��ش����⡣��10�֣�
�� 1982��Ĵ�����ѧ�ߴ�θ�����֯�з���������ݸ˾���

61. �����ݸ˾����Ŵ����ʼ��зֲ��������Ϊ���������� ��
62. ��ͼ4֧�Թֱܷ����4�������ڰ��������������֬����3.5g/L���е�����״̬�����Тں��Թܴ��������ݸ˾�������״̬����ͼ�жϣ��þ������������������� �����²���������������ϸ��������״̬�������Թ����������� ������
63. �±���ij���������䷽��
| �ɷ� | ������ | KH2PO4 | MgSO4 | NaCl | CaSO4 | CaCO3 | ��֬ | ����ˮ |
| ���� | 10g | 0.2g | 0.2g | 0.2g | 0.2g | 5g | 3.5g | 1L |
�������ݸ˾����ֵ�pH���˵ĸ��������У�����37��������һ��ʱ����ڸ��������������ݸ˾�����Ŀ�ȸս���ʱ���������� ����Ҫԭ���ǣ����������������������� ��
�����ݸ˾���̬����ͼ��ʾ���þ��������п�����θ�����θ��������
64. �����ݸ˾�����������pHΪ6��7������θǻ��pH��1��2֮�䣬��θճĤ��ճҺ�㿿����Ƥϸ����pHΪ7.4���ҡ��������ݸ˾���ʳ�����θǻ�������ṹ�ص��Լ��ܵ���θ��������ԣ��Ʋ�þ���θ����δ�
��������������������������������������������������������������������������
��������������������������������������������������������������������������
65. ���ݵ�ʮ������Ϣ���������ݸ˾��Ƿ����ڹ�ϸ�������������� ��
ԭ�������������������� ������������������������������
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
��ʮ�������й���������ϣ��ش����⡣
1982��Ĵ�����ѧ�ߴ�θ�����֯�з���������ݸ˾���
61. �����ݸ˾����Ŵ����ʼ��зֲ��������Ϊ ��

62. ��ͼ4֧�Թֱܷ����4�������ڰ��������������֬����3.5g/L���е�����״̬�����Тں��Թܴ��������ݸ˾�������״̬����ͼ�жϣ��þ��� �����²���������������ϸ��������״̬�������Թ� ������
63. �±���ij���������䷽��
| �ɷ� | ������ | KH2PO4 | MgSO4 | NaCl | CaSO4 | CaCO3 | ��֬ | ����ˮ |
| ���� | 10g | 0.2g | 0.2g | 0.2g | 0.2g | 5g | 3.5g | 1L |
�������ݸ˾����ֵ�pH���˵ĸ��������У�����37��������һ��ʱ����ڸ��������������ݸ˾�����Ŀ�ȸս���ʱ ����Ҫԭ���ǣ� ��
�����ݸ˾���̬����ͼ��ʾ���þ��������п�����θ�����θ��������

64. �����ݸ˾�����������pHΪ6��7������θǻ��pH��1��2֮�䣬��θճĤ��ճҺ�㿿����Ƥϸ����pHΪ7.4���ҡ��������ݸ˾���ʳ�����θǻ�������ṹ�ص��Լ��ܵ���θ��������ԣ��Ʋ�þ���θ����δ�
65. ���ݵ�ʮ������Ϣ���������ݸ˾��Ƿ����ڹ�ϸ���� ��
ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010��߿���������������֮ר��ʮ�� ���\��ʵ�� ���ͣ��ۺ���
��ʮ�������й���������ϣ��ش����⡣��10�֣�
1982��Ĵ�����ѧ�ߴ�θ�����֯�з���������ݸ˾���
61. �����ݸ˾����Ŵ����ʼ��зֲ��������Ϊ ��
62. ��ͼ4֧�Թֱܷ����4�������ڰ��������������֬����3.5g/L���е�����״̬�����Тں��Թܴ��������ݸ˾�������״̬����ͼ�жϣ��þ��� �����²���������������ϸ��������״̬�������Թ� ������
63. �±���ij���������䷽��
| �ɷ� | ������ | KH2PO4 | MgSO4 | NaCl | CaSO4 | CaCO3 | ��֬ | ����ˮ |
| ���� | 10g | 0.2g | 0.2g | 0.2g | 0.2g | 5g | 3.5g | 1L |

�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010��ȫ����ͨ�ߵ�ѧУ����ͳһ���ԣ��Ϻ�����������ѧ�Ծ� ���ͣ��ۺ���
��ʮ�������й���������ϣ��ش����⡣��10�֣�
1982��Ĵ�����ѧ�ߴ�θ�����֯�з���������ݸ˾���
61. �����ݸ˾����Ŵ����ʼ��зֲ��������Ϊ ��

62. ��ͼ4֧�Թֱܷ����4�������ڰ��������������֬����3.5g/L���е�����״̬�����Тں��Թܴ��������ݸ˾�������״̬����ͼ�жϣ��þ��� �����²���������������ϸ��������״̬�������Թ� ������
63. �±���ij���������䷽��
|
�ɷ� |
������ |
KH2PO4 |
MgSO4 |
NaCl |
CaSO4 |
CaCO3 |
��֬ |
����ˮ |
|
���� |
10g |
0.2g |
0.2g |
0.2g |
0.2g |
5g |
3.5g |
1L |
�������ݸ˾����ֵ�pH���˵ĸ��������У�����37��������һ��ʱ����ڸ��������������ݸ˾�����Ŀ�ȸս���ʱ ����Ҫԭ���ǣ� ��
�����ݸ˾���̬����ͼ��ʾ���þ��������п�����θ�����θ��������

64. �����ݸ˾�����������pHΪ6��7������θǻ��pH��1��2֮�䣬��θճĤ��ճҺ�㿿����Ƥϸ����pHΪ7.4���ҡ��������ݸ˾���ʳ�����θǻ�������ṹ�ص��Լ��ܵ���θ��������ԣ��Ʋ�þ���θ����δ�
65. ���ݵ�ʮ������Ϣ���������ݸ˾��Ƿ����ڹ�ϸ���� ��
ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
�����й���������ϣ��ش����⡣
1982��Ĵ�����ѧ�ߴ�θ�����֯�з���������ݸ˾���
1�������ݸ˾����Ŵ����ʼ��зֲ��������Ϊ ��

2����ͼ4֧�Թֱܷ����4�������ڰ��������������֬����3��5g/L���е�����״̬�����Тں��Թܴ��������ݸ˾�������״̬����ͼ�жϣ��þ��� �����²���������������ϸ��������״̬�������Թ� ������
3���±���ij���������䷽��
| �ɷ� | ������ | KH2PO4 | MgSO4 | NaCl | CaSO4 | CaCO3 | ��֬ | ����ˮ |
| ���� | 10g | 0��2g | 0��2g | 0��2g | 0��2g | 5g | 3��5g | 1L |
�������ݸ˾����ֵ�pH���˵ĸ��������У�����37��������һ��ʱ����ڸ��������������ݸ˾�����Ŀ�ȸս���ʱ ����Ҫԭ���ǣ� ��
�����ݸ˾���̬��ͼ��ʾ���þ��������п�����θ�����θ��������

4�������ݸ˾�����������pHΪ6��7������θǻ��pH��1��2֮�䣬��θճĤ��ճҺ�㿿����Ƥϸ����pHΪ7��4���ҡ��������ݸ˾���ʳ�����θǻ�������ṹ�ص��Լ��ܵ���θ��������ԣ��Ʋ�þ���θ����δ�
5����������������Ϣ���������ݸ˾��Ƿ����ڹ�ϸ���� ��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
�����й���������ϣ��ش����⡣
1982��Ĵ�����ѧ�ߴ�θ�����֯�з���������ݸ˾���
(1)�����ݸ˾����Ŵ����ʼ��зֲ��������Ϊ________��

(2)��ͼ4֧�Թֱܷ����4�������ڰ����������(��֬����3.5 g/L)�е�����״̬�����Тں��Թܴ��������ݸ˾�������״̬����ͼ�жϣ��þ���________�����²���������������ϸ��������״̬�������Թ�________������
(3)�±���ij���������䷽��
| �ɷ� | ���� |
| ������ | 10 g |
| KH2PO4 | 0.2 g |
| MgSO4 | 0.2 g |
| NaCl | 0.2 g |
| CaSO4 | 0.2 g |
| CaCO3 | 5 g |
| ��֬ | 3.5 g |
| ����ˮ | 1 L |
�������ݸ˾����ֵ�pH���˵ĸ��������У�����37 ��������һ��ʱ����ڸ��������������ݸ˾�����Ŀ�ȸս���ʱ________����Ҫԭ���ǣ� ________________________��
�����ݸ˾���̬��ͼ��ʾ���þ��������п�����θ�����θ��������
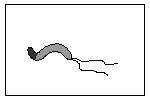
(4)�����ݸ˾�����������pHΪ6��7������θǻ��pH��1��2֮�䣬��θ�Ĥ���Һ�㿿����Ƥϸ����pHΪ7.4���ҡ��������ݸ˾���ʳ�����θǻ�������ṹ�ص��Լ��ܵ���θ��������ԣ��Ʋ�þ���θ����δ� ________________________________��
(5)������������Ϣ���������ݸ˾��Ƿ����ڹ�ϸ����________��ԭ����_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com