
��ĩֲ���ҶƬ˥��ʱ����Ҷ��������ʼ�γ���㣨��ͼ����ʾ���������ֲ���������䡣��㲿λϸ����ص��������ͼ����ʾ�����ͼ�ش��������⣺
 ��1������ͼ��֪��Ҷ���������IJ�������ߣߣߣߣ�ֲ�D�أ������й�ϵ��
��1������ͼ��֪��Ҷ���������IJ�������ߣߣߣߣ�ֲ�D�أ������й�ϵ��
��2��A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬���ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ��ϸ��Ĥ�ģߣߣߣ����ã����ڵ�ϸ���⡣
��3���ù��̷��ڵ�ϸ����֮������ʣ�������γ�����������ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4����һ�����о�������Ҷ�����������γɣ�������������������صIJ��룬��˵���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��5���澳��ָ��ֲ������˺��Ļ���������������պɺ��ȡ�ֲ����澳�ĵֿ������Dz�һ���ģ����Ŵ����غ�������ص�˫��Ӱ�졣ֲ����澳�Ŀ���ǿ����ֲ��ĵ絼�ʵͣ���֮���絼�ʾߡ��絼�ʿ�����һ���ļ����ֶβ��������������Ϊ������������ֲ����澳�ĵֿ�������ʹ�����в������һ��ʵ�飬��̽���������ֲ����Ե�Ӱ�졣������Ʒ������������ƿ��״̬������һ�µĻƹ�����10�ꡢ����Ũ�ȵ������ᡢ�ⶨ�絼�ʵ�װ�á�0.25mol��L NaCl��Һ������ˮ��
��ʵ�鲽�裺
��һ������10��ƹ�����ƽ���ֳɼס������飻
�ڶ������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
���������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
���IJ����ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Ԥ�ڽ�������ۣ��ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��1����ϩ ��2������ ��3��������ά��ø����ά��ˮ�⣬ʹҶ������ϸ��֮�����ϵ�����γ���� ��4��ֲ��������ĵ��ڣ����ɶ��ּ����Э����ͬ���ڵ�
��5����ʵ�鲽�裺
�ڶ�������ס�������ƹ�������������зֱ���������������0.25mol/L NaCl��Һ��
���������������ʩ��������������Һ����������ʩ������ˮ��
���IJ����ֱ�ⶨ�ס�������ĵ絼��
��Ԥ�ڽ������ۣ�
����ס�������ĵ絼����ȣ���˵���������ı�ֲ����澳�ĵֿ�����
�������絼�ʴ�������ĵ絼�ʣ���˵��������ή��ֲ����澳�ĵֿ�����
�������絼��С������ĵ絼�ʣ���˵�������������ֲ����澳�ĵֿ�
��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
��ĩֲ���ҶƬ˥��ʱ����Ҷ��������ʼ�γ���㣨��ͼ����ʾ���������ֲ���������䡣��㲿��ϸ����ص��������ͼ����ʾ�����ͼ�ش��������⣺
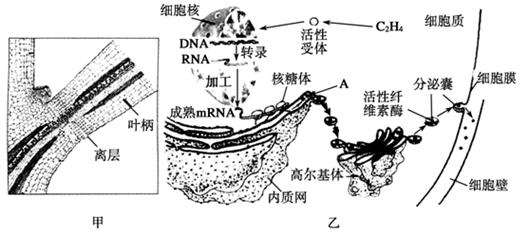
��1����ͼ�ҿ�֪��Ҷ���������IJ������� ��ֲ�D�أ������й�ϵ���ڸ�ֲ�D�ص������£�ϸ�����е��йػ�����б������ı�����̵ij����ֱ��� �� ��
��2��A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬���ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ��ϸ���� ���ã����ڵ�ϸ���⡣��һϵ�еĹ���֤�� �� ��
��3����һ�����о�������Ҷ�����������γɣ����������������ֲ�D�صIJ��룬��˵�� ��
��4��˥��ҶƬ��N��P��������Ҷ�к��� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
��ĩֲ���ҶƬ˥��ʱ����Ҷ��������ʼ�γ����(��ͼ����ʾ)�������ֲ���������䡣��㲿λϸ����ص��������ͼ����ʾ�����ͼ�ش��������⣺

(1)��ͼ�ҿ�֪��Ҷ���������IJ�������________(ֲ�D��)�����й�ϵ���ڸ�ֲ�D�ص������£�![]() ϸ�����е��йػ�����б�����������̷ֱ���________��________�н��С�
ϸ�����е��йػ�����б�����������̷ֱ���________��________�н��С�
(2)A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬���ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ��ϸ��Ĥ��________���ã����ڵ�ϸ���⡣��һϵ�еĹ����ܹ�֤��____________________________________��
(3)�ù��̷��ڵ�ϸ����֮������ʣ�������γ������������ʲô��
(4)��ͼ���п��Կ��������������mRNA���ӣ���һ����Ҳ����������ϸ���У���ͼ��ʾ��������ѧ����������mRNA�ǵ������ṹ________�����ױ�ˮ���____________�������������m![]() RNA���ӣ����ܹ��ڶ�ʱ���ڣ�Ѹ�ٺϳɽ϶�IJ���������Լ������������ģ����ϡ���Լ����ԭ��
RNA���ӣ����ܹ��ڶ�ʱ���ڣ�Ѹ�ٺϳɽ϶�IJ���������Լ������������ģ����ϡ���Լ����ԭ��

(5)��һ�����о�������Ҷ�����������γɣ�������������������صIJ��룬��˵����ʲô��
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010�콭����ͨͨ������У���������Ծ��������� ���ͣ��ۺ���
��ĩֲ���ҶƬ˥��ʱ�� ��Ҷ��������ʼ�γ���㣨��ͼ����ʾ���������ֲ���������䡣��㲿λϸ����ص��������ͼ����ʾ�����ͼ�ش�����
��Ҷ��������ʼ�γ���㣨��ͼ����ʾ���������ֲ���������䡣��㲿λϸ����ص��������ͼ����ʾ�����ͼ�ش����� ���⣺
���⣺ ��1������ͼ��֪��Ҷ���������IJ�������ߣߣߣߣ�ֲ�D�أ������й�ϵ��
��1������ͼ��֪��Ҷ���������IJ�������ߣߣߣߣ�ֲ�D�أ������й�ϵ��
�� 2��A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬��
2��A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬�� �ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ��ϸ��Ĥ�ģߣߣߣ����ã����ڵ�ϸ���⡣
�ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ��ϸ��Ĥ�ģߣߣߣ����ã����ڵ�ϸ���⡣
��3���ù��̷��ڵ�ϸ����֮������ʣ�������γ�����������ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4����һ�����о�������Ҷ�����������γɣ�������������������صIJ��룬��˵���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��5���澳��ָ��ֲ������˺��Ļ���������������պɺ��ȡ�ֲ����澳�ĵֿ������Dz�һ���ģ����Ŵ����غ�������ص�˫��Ӱ�졣ֲ����澳�Ŀ���ǿ����ֲ��ĵ絼�ʵͣ���֮���絼�ʾߡ��絼�ʿ�����һ���ļ� ���ֶβ��������������Ϊ������������ֲ����澳�ĵֿ�������ʹ�����в������һ��ʵ�飬��̽���������ֲ����Ե�Ӱ�졣������Ʒ������������ƿ��״̬������һ�µĻƹ�����10�ꡢ����Ũ�ȵ������ᡢ�ⶨ�絼�ʵ�װ�á�0.25mol��L NaCl��Һ������ˮ��
���ֶβ��������������Ϊ������������ֲ����澳�ĵֿ�������ʹ�����в������һ��ʵ�飬��̽���������ֲ����Ե�Ӱ�졣������Ʒ������������ƿ��״̬������һ�µĻƹ�����10�ꡢ����Ũ�ȵ������ᡢ�ⶨ�絼�ʵ�װ�á�0.25mol��L NaCl��Һ������ˮ��
��ʵ�鲽�裺
��һ������10��ƹ�����ƽ���ֳɼס������飻 �ڶ������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
�ڶ������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
���������ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ�
���IJ����ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Ԥ�ڽ�������ۣ��ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ�����ʡ�����и�����ѧ�ڵ��Ĵ��¿������ۣ����ﲿ�� ���ͣ��ۺ���
��ĩֲ���ҶƬ˥��ʱ����Ҷ��������ʼ�γ���㣨��ͼ����ʾ���������ֲ���������䡣��㲿��ϸ����ص��������ͼ����ʾ�����ͼ�ش��������⣺

��1����ͼ�ҿ�֪��Ҷ���������IJ������� ��ֲ�D�أ������й�ϵ���ڸ�ֲ�D�ص������£�ϸ�����е��йػ�����б������ı�����̵ij����ֱ��� �� ��
��2��A�������������Գ�ѿ�ķ�ʽ�γ�С�ݣ������߶����壬���ɸ߶������γɷ����ң�����ϸ��Ĥ������ͨ��ϸ���� ���ã����ڵ�ϸ���⡣��һϵ�еĹ���֤�� �� ��
��3����һ�����о�������Ҷ�����������γɣ����������������ֲ�D�صIJ��룬��˵�� ��
��4��˥��ҶƬ��N��P��������Ҷ�к��� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com