
ʵ����
��һ�����ڡ���֤��е���غ㶨�ɡ���ʵ����
��1���������ij�����ʱ����У�A������Դ��Bֱ����Դ��Cֽ����D�����ӵ����E�̶ȳߡ�F��ƽ��G����̨�����и�ʵ�鲻��Ҫ��������______________________��
��2��ʵ���У���Ҫ�����ͼ�����������ǣ��� ��
A������ĸ߶� B���������ٶ�
C���ش������� D���ش��������ٶ�
��3��ʵ���У�������ʱ��û����ȫ��ֱ��������ֽ��ͨ����λ��ʱ�ܵ�������ͬʱ����Ҳ���������ã���Ƚ�����ʱ�����С���������ܺ����ӵĶ��ܣ�Ӧ�Ц�EP________��EK���������������������
��4��ʵ���У����ȡ����������ϴ���ش���������С���ش���ʵ�飬��Ƚ���������¼�С���������ܦ�EP�����ӵĶ��ܦ�EK��ѡ������___________����ϴ�С�������ش���ʵ��ʱ�����ֵ��EK/��EP�ϴ�
������������֤��е���غ㶨�ɡ���ʵ�����������������ķ�������������λ��Ч���֣�
��1����ʵ������ʹ�õ���������Ϊm��0.1kg�����ֽ����ͼ��ʾ��O�ǵ�һ�㣬OA�仹��δ�����ĵ㣬����ʱ���Ϊ0.02s�����¼B��ʱ��������ٶ�vB�� ������Ķ���ΪEK�� ���ӿ�ʼ���䵽B�㣬������������ܸı����Ǧ�EP�� ����ˣ����Եó��Ľ����ǣ� ��
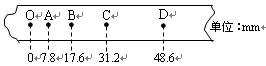
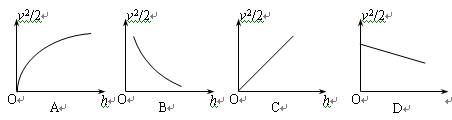
��2������ֽ�������ظ�����ٶ�v����������ľ���h������v2/2Ϊ���ᣬ��hΪ���ử����ͼ��Ӧ����ͼ�еģ��� ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��Ķ�����
4��2n2(l+
| ||
| t2 |
4��2n2(l+
| ||
| t2 |


�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
ʵ���⣨18�֣�
��һ�����ڡ���֤��е���غ㶨�ɡ���ʵ����
��1���������ij�����ʱ����У�A������Դ��Bֱ����Դ��Cֽ����D�����ӵ����E�̶ȳߡ�F��ƽ��G����̨�����и�ʵ�鲻��Ҫ��������______________________��
��2��ʵ���У���Ҫ�����ͼ�����������ǣ��� ��
A������ĸ߶� B���������ٶ�
C���ش������� D���ش��������ٶ�
��3��ʵ���У�������ʱ��û����ȫ��ֱ��������ֽ��ͨ����λ��ʱ�ܵ�������ͬʱ����Ҳ���������ã���Ƚ�����ʱ�����С���������ܺ����ӵĶ��ܣ�Ӧ�Ц�EP________��EK���������������������
��4��ʵ���У����ȡ����������ϴ���ش���������С���ش���ʵ�飬��Ƚ���������¼�С���������ܦ�EP�����ӵĶ��ܦ�EK��ѡ������___________����ϴ�С�������ش���ʵ��ʱ�����ֵ��EK/��EP�ϴ�
������������֤��е���غ㶨�ɡ���ʵ�����������������ķ�������������λ��Ч���֣�
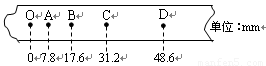
��1����ʵ������ʹ�õ���������Ϊm��0.1kg�����ֽ����ͼ��ʾ��O�ǵ�һ�㣬OA�仹��δ�����ĵ㣬����ʱ���Ϊ0.02s�����¼B��ʱ��������ٶ�vB�� ������Ķ���ΪEK�� ���ӿ�ʼ���䵽B�㣬������������ܸı����Ǧ�EP�� ����ˣ����Եó��Ľ����ǣ� ��
��2������ֽ�������ظ�����ٶ�v����������ľ���h������v2/2Ϊ���ᣬ��hΪ���ử����ͼ��Ӧ����ͼ�еģ��� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010��㶫ʡտ�����и�һ�ڶ�ѧ����ĩ���ԣ����ۣ��������� ���ͣ�ʵ����
ʵ���⣨18�֣�
��һ�����ڡ���֤��е���غ㶨�ɡ���ʵ����
��1���������ij�����ʱ����У�A������Դ��Bֱ����Դ��Cֽ����D�����ӵ����E�̶ȳߡ�F��ƽ��G����̨�����и�ʵ�鲻��Ҫ��������______________________��
��2��ʵ���У���Ҫ�����ͼ�����������ǣ��� ��
| A������ĸ߶� | B���������ٶ� |
| C���ش������� | D���ش��������ٶ� |


�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010��㶫ʡ��һ�ڶ�ѧ����ĩ���ԣ����ۣ��������� ���ͣ�ʵ����
ʵ���⣨18�֣�
��һ�����ڡ���֤��е���غ㶨�ɡ���ʵ����
��1���������ij�����ʱ����У�A������Դ��Bֱ����Դ��Cֽ����D�����ӵ����E�̶ȳߡ�F��ƽ��G����̨�����и�ʵ�鲻��Ҫ��������______________________��
��2��ʵ���У���Ҫ�����ͼ�����������ǣ��� ��
A������ĸ߶� B���������ٶ�
C���ش������� D���ش��������ٶ�
��3��ʵ���У�������ʱ��û����ȫ��ֱ��������ֽ��ͨ����λ��ʱ�ܵ�������ͬʱ����Ҳ���������ã���Ƚ�����ʱ�����С���������ܺ����ӵĶ��ܣ�Ӧ�Ц�EP________��EK���������������������
��4��ʵ���У����ȡ����������ϴ���ش���������С���ش���ʵ�飬��Ƚ���������¼�С���������ܦ�EP�����ӵĶ��ܦ�EK��ѡ������___________����ϴ�С�������ش���ʵ��ʱ�����ֵ��EK/��EP�ϴ�
������������֤��е���غ㶨�ɡ���ʵ�����������������ķ�������������λ��Ч���֣�
��1����ʵ������ʹ�õ���������Ϊm��0.1kg�����ֽ����ͼ��ʾ��O�ǵ�һ�㣬OA�仹��δ�����ĵ㣬����ʱ���Ϊ0.02s�����¼B��ʱ��������ٶ�vB�� ������Ķ���ΪEK�� ���ӿ�ʼ���䵽B�㣬������������ܸı����Ǧ�EP�� ����ˣ����Եó��Ľ����ǣ� ��
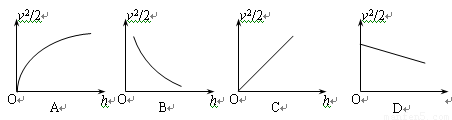
��2������ֽ�������ظ�����ٶ�v����������ľ���h������v2/2Ϊ���ᣬ��hΪ���ử����ͼ��Ӧ����ͼ�еģ��� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com