
 ��1��Ϊ�˽��������Դ֮�裬ʵ���ú��ܴ���ú��ʯ�͵Ȳ���������Դ���ܶ���Ҷ�������ȫ�����˾۱䡰����̫���������ǴӺ�ˮ����ȡԭ�ϣ������ڶȵĸ����·����Ŀɿغ˾۱䷴Ӧ����ѧ�����ݵĺ˷�Ӧ������
��1��Ϊ�˽��������Դ֮�裬ʵ���ú��ܴ���ú��ʯ�͵Ȳ���������Դ���ܶ���Ҷ�������ȫ�����˾۱䡰����̫���������ǴӺ�ˮ����ȡԭ�ϣ������ڶȵĸ����·����Ŀɿغ˾۱䷴Ӧ����ѧ�����ݵĺ˷�Ӧ������| E1 |
| n2 |
| 3 |
| 16 |
| 3 |
| 4 |
| 1 |
| 4 |
| 3 |
| 4 |
| E1 |
| 4 |
| E1 |
| n2 |
| 1 |
| 4 |
| 3 |
| 4 |
| E1 |
| 4 |
| E1 |
| 4 |
| 3 |
| 16 |
| E1 |
| 16 |
| E1 |
| n2 |
| 1 |
| 4 |
| 1 |
| 2 |
| 1 |
| 4 |
| 1 |
| 2 |
| 1 |
| 2 |
| ||
| m2 |
| ||
| m2 |
| ||
| m2 |
| ||
| m2 |


 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д� �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ�����������ѧ����1���¿��������Ծ� ���ͣ�ѡ����
Ϊ�˽��������Դ֮�裬ʵ���ú��ܴ���ú��ʯ�͵Ȳ���������Դ���ܶ���Ҷ�������ȫ�����˾۱䡰����̫���������ǴӺ�ˮ����ȡԭ�ϣ������ڶȵĸ����·����Ŀɿغ˾۱䷴Ӧ����ѧ�����ݵĺ˷�Ӧ�����ǣ�
A�� Th�� Pa�� e B�� U�� n�� Ba�� Kr��3 n
C�� H�� H�� He�� n D�� U�� Th�� He
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ�걱���з�ɽ��������һѧ����ĩ���������Ծ� ���ͣ�ѡ����
Ϊ�˽��������Դ֮�裬ʵ���ú��ܴ���ú��ʯ�͵Ȳ���������Դ���ܶ���Ҷ�������ȫ�����˾۱䡰����̫���������ǴӺ�ˮ����ȡԭ�ϣ������ڶȵĸ����·����Ŀɿغ˾۱䷴Ӧ����ѧ�����ݵĺ˷�Ӧ������
A��
B��
C��
D��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ�����ʡ��ɳ������һ�и������£����ߴ��¿������Ծ��������棩 ���ͣ������
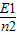 ��n=1��2��������E1��n=1ʱ�Ļ�̬��������һ��ԭ�ӷ�������Ϊ-
��n=1��2��������E1��n=1ʱ�Ļ�̬��������һ��ԭ�ӷ�������Ϊ-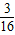 E1�Ĺ��Ӻ��ڱȻ�̬�����߳�-
E1�Ĺ��Ӻ��ڱȻ�̬�����߳�- E1�ļ���̬������ԭ�ӷ������ǰ���ڵ�______�ܼ���������Ӻ��ڵ�______�ܼ���
E1�ļ���̬������ԭ�ӷ������ǰ���ڵ�______�ܼ���������Ӻ��ڵ�______�ܼ��� ���ɵ�һ�λָ���ԭ��ʱB���ٶȣ�
���ɵ�һ�λָ���ԭ��ʱB���ٶȣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com