
题目列表(包括答案和解析)
1mol碳完全燃烧后可放出393.5KJ的热量,下列热化学方程式正确的是( )
| A.C(固)+O2(气)=CO2(气); △H ="+393.5" kJ/mol |
B.C(固)+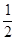 O2(气)=CO(气); △H ="-393.5" kJ/mol O2(气)=CO(气); △H ="-393.5" kJ/mol |
| C.C + O2= CO2;△H ="-393.5" kJ/mol |
| D.C(固)+O2(气)=CO2(气); △H ="-393.5" kJ/mol |
1mol碳完全燃烧后可放出393.5KJ的热量,下列热化学方程式正确的是( )
A.C(固)+O2(气)=CO2(气); △H ="+393.5" kJ/mol
B.C(固)+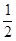 O2(气)=CO(气);
△H ="-393.5" kJ/mol
O2(气)=CO(气);
△H ="-393.5" kJ/mol
C.C + O2= CO2;△H ="-393.5" kJ/mol
D.C(固)+O2(气)=CO2(气); △H ="-393.5" kJ/mol
1mol碳完全燃烧后可放出393.5KJ的热量,下列热化学方程式正确的是:
A.C(s)+O2(g)=CO2(g); △H = +393.5 kJ•mol-1
B.C(s)+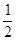 O2(g)=CO(g);
△H = -393.5 kJ•mol-1
O2(g)=CO(g);
△H = -393.5 kJ•mol-1
C.C+O2=CO2 ; △H = -393.5 kJ•mol-1
D.C(s)+O2(g)=CO2(g); △H = -393.5 kJ•mol-1
| A.C(固)+O2(气)=CO2(气); △H ="+393.5" kJ/mol |
B.C(固)+ O2(气)=CO(气); △H ="-393.5" kJ/mol O2(气)=CO(气); △H ="-393.5" kJ/mol |
| C.C + O2= CO2;△H ="-393.5" kJ/mol |
| D.C(固)+O2(气)=CO2(气); △H ="-393.5" kJ/mol |
1mol碳完全燃烧后可放出393.5KJ的热量,下列热化学方程式正确的是:
A.C(s)+O2(g)=CO2(g); △H = +393.5 kJ??mol-1
B.C(s)+![]() O2(g)=CO(g); △H = -393.5 kJ??mol-1
O2(g)=CO(g); △H = -393.5 kJ??mol-1
C.C+O2=CO2 ; △H = -393.5 kJ??mol-1
D.C(s)+O2(g)=CO2(g); △H = -393.5 kJ??mol-1
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com