
题目列表(包括答案和解析)
A.![]() N2H4(g)+
N2H4(g)+![]() O2 (g)====
O2 (g)====![]() N2 (g)+ H2O (g);ΔH=+267kJ/mol
N2 (g)+ H2O (g);ΔH=+267kJ/mol
B.N2H4(g)+O2 (g)+====N2(g)+2H2O (g);ΔH=-133.5 kJ/mol
C.N2H4(g)+O2 (g)==== N2 (g)+2H2O (g);ΔH=+534 kJ/mol
D.N2H4(g)+O2 (g)==== N2 (g)+2H2O (g);ΔH=-534 kJ/mol
N2H4是一种高效清洁的火箭燃料。0.25 mol N2H4(g)完全燃烧生成氮气和气态水时,放出133.5 kJ热量。则下列热化学方程式中正确的是( )
A.1/2 N2H4(g)+1/2O2(g)===1/2 N2(g)+H2O(g) ΔH=+267 kJ·mol-1
B.N2H4(g)+O2(g)===N2(g)+2H2O(l) ΔH=-133.5 kJ·mol-1
C.N2H4(g)+O2(g)===N2(g)+2H2O(g) ΔH=+534 kJ·mol-1
D.N2H4(g)+O2(g)===N2(g)+2H2O(g) ΔH=-534 kJ·mol-1
N2H4是一种高效清洁的火箭燃料。0.25 mol N2H4(g)完全燃烧生成氮气和气态水时,
放出133.5 kJ热量。则下列热化学方程式中正确的是( )
A.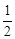 N2H4(g)+
N2H4(g)+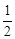 O2(g)====
O2(g)====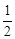 N2(g)+H2O(g);ΔH=+267
kJ·mol-1
N2(g)+H2O(g);ΔH=+267
kJ·mol-1
B.N2H4(g)+O2(g)====N2(g)+2H2O(g);ΔH =-534 kJ·mol-1
C.N2H4(g)+O2(g)====N2(g)+2H2O(g);ΔH =+534 kJ·mol-1
D.N2H4(g)+O2(g)====N2(g)+2H2O(l);ΔH =-133.5 kJ·mol-1
N2H4是一种高效清洁的火箭燃料。0.25 mol N2H4(g)完全燃烧生成氮气和气态水时,放出133.5 kJ热量 。则下列热化学方程式中正确的是( )
A.N2H4(g)+O2(g)===N2(g)+2H2O(g) ΔH=+267 kJ·mol-1
B.N2H4(g)+O2(g)===N2(g)+2H2O(g) ΔH=-534 kJ·mol-1
C.N2H4(g)+O2(g)===N2(g)+2H2O(g) ΔH=+534 kJ·mol-1
D.N2H4(g)+O2(g)===N2(g)+2H2O(l) ΔH=-133.5 kJ·mol-1
N2H4是一种高效清洁的火箭燃料。0.25 mol N2H4(g)完全燃烧生成氮气和气态水时,放出133.5 kJ热量。则下列热化学方程式中正确的是
A.N2H4(g)+O2(g)===N2(g)+H2O(g) ΔH=+267 kJ·mol-1
B.N2H4(g)+O2(g)===N2(g)+2H2O(l) ΔH=-133.5 kJ·mol-1
C.N2H4(g)+O2(g)===N2(g)+2H2O(g) ΔH=+534 kJ·mol-1
D.N2H4(g)+O2(g)===N2(g)+2H2O(g) ΔH=-534 kJ·mol-1
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com