
题目列表(包括答案和解析)
已知⑴H2(g)+ 1/2 O2(g)=H2O(g); △H1=a kJ/mol
⑵2H2(g)+ O2(g)=2H2O(g); △H2=b kJ/mol
⑶H2(g)+ 1/2O2(g)=H2O(l); △H3=c kJ/mol
⑷2H2(g)+ O2(g)=2H2O(l); △H4=d kJ/mol
下列关系式中正确的是( )
A.a<c<0 B.b>d>0 C.2a=b<0 D.2c=d>0
已知下列热化学方程式:
(1)CH3COOH(l)+2O2(g)==2CO2(g)+2H2O(l) ΔH1=-870.3 kJ·mol-1
(2)C(s)+O2(g)===CO2(g) ΔH2=-393.5 kJ·mol-1
(3)H2(g)+ O2(g)===H2O(l) ΔH3=-285.8 kJ·mol-1,
O2(g)===H2O(l) ΔH3=-285.8 kJ·mol-1,
则反应2C(s)+2H2(g)+O2(g)===CH3COOH(l)的焓变ΔH为
| A.+ 488.3 kJ·mol-1 | B.-244.15 kJ·mol-1 |
| C.+ 244.15 kJ·mol-1 | D.-488.3 kJ·mol-1 |
已知氢气和碳燃烧的热化学方程式为:
①2H2(g)+O2(g)=2H2O(l) △H1=-akJ·mol-1
②H2(g)+1/2O2(g)=H2O(g) △H2=-bkJ·mol-1
③C(s)+1/2O2(g)=CO(g) △H3=-ckJ·mol-1
④C(s)+O2(g)=CO2(g) △H4=-dkJ·mol-1
下列说法错误的是
A.氢气的燃烧热为△H=-akJ·mol-1 B.c<d
C.一氧化碳的燃烧热为△H=-(d-c)kJ·mol-1 D.0.5a>b
已知下列热化学方程式:
(1)CH3COOH(l)+2O2(g)==2CO2(g)+2H2O(l) ΔH1=-870.3 kJ·mol-1
(2)C(s)+O2(g)===CO2(g) ΔH2=-393.5 kJ·mol-1
(3)H2(g)+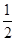 O2(g)===H2O(l)
ΔH3=-285.8 kJ·mol-1,
O2(g)===H2O(l)
ΔH3=-285.8 kJ·mol-1,
则反应2C(s)+2H2(g)+O2(g)===CH3COOH(l)的焓变ΔH为
A.+ 488.3 kJ·mol-1 B.-244.15 kJ·mol-1
C.+ 244.15 kJ·mol-1 D.-488.3 kJ·mol-1
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com