
7. (07全国理综卷II)已知:①1 mol H2分子中化学键断裂时需要吸收436 kJ的能量
②1 mol Cl2分子中化学键断裂时需要吸收243 kJ的能量
③由H原子和Cl原子形成1 mol HCl分子时释放431 kJ的能量
下列叙述正确的是( )
(A)氢气和氯气反应生成氯化氢气体的热化学方程式是 H2(g)+Cl2(g) = 2HCl(g)
(B)氢气和氯气反应生成2 mol氯化氢气体,反应的DH = 183 kJ/mol
(C)氢气和氯气反应生成2 mol氯化氢气体,反应的DH =-183 kJ/mol
(D)氢气和氯气反应生成1 mol氯化氢气体,反应的DH =-183 kJ/mol
6.(07上海卷)已知:CH3CH2CH2CH3(g)+6.5O2(g) 4CO2(g)+5H2O(l);DH =-2878 kJ
4CO2(g)+5H2O(l);DH =-2878 kJ
(CH3)2CHCH3(g)+6.5O2(g) 4CO2(g)+5H2O(l);DH =-2869 kJ
4CO2(g)+5H2O(l);DH =-2869 kJ
下列说法正确的是( )
(A)正丁烷分子储存的能量大于异丁烷分子
(B)正丁烷的稳定性大于异丁烷
(C)异丁烷转化为正丁烷的过程是一个放热过程
(D)异丁烷分子中的碳氢键比正丁烷的多
5.(07江苏卷)甲醇质子交换膜燃料电池中将甲醇蒸气转化为氢气的两种反应原理是
①CH3OH(g)+H2O(g) = CO2(g)+3H2(g);DH = + 49.0 kJ·mol-1
②CH3OH(g)+1/2O2(g) = CO2(g)+2H2(g);DH =-192.9 kJ·mol-1
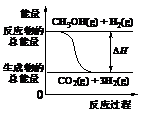
下列说法正确的是( )
(A)CH3OH的燃烧热为192.9 kJ·mol-1
(B)反应①中的能量变化如图所示
(C)CH3OH转变成H2的过程一定要吸收能量
(D)根据②推知反应:CH3OH(l)+1/2O2(g) = CO2(g)+2H2(g)的H>-192.9 kJ·mol-1
4.(07广东化学卷)灰锡以粉末状存在)和白锡是锡的两种同素异形体。
已知:①Sn(s、白)+2HCl(aq)=SnCl2(aq)+H2(g);DH1
②Sn(s、灰)+2HCl(aq)=SnCl2(aq)+H2(g);DH2
③Sn(s、灰)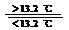 Sn(s、白);DH3=+2.1 kJ/mol
Sn(s、白);DH3=+2.1 kJ/mol
下列说法正确的是( )
(A)DH1>DH2
(B)锡在常温下以灰锡状态存在
(C)灰锡转化为白锡的反应是放热反应
(D)锡制器皿长期处于低于13.2 ℃的环境中,会自行毁坏
3.(08四川卷)下列关于热化学反应的描述中正确的是( )
A.HCl和NaOH反映的中和热△H=-57.3kJ/mol,则H2SO4和Ca(OH)2反映的中和热△H=2×(-57.3)kJ/mol
B.CO(g)的燃烧热是283.0kJ/mol,则2CO2(g)===2CO(g)+O2(g)反应的△H=2×283.0kJ/mol
C.需要加热才能发生的反应一定是吸热反应
D.1mol甲烷燃烧生成气态水和二氧化碳所放出的热量是甲烷的燃烧热
2.(08广东卷)下列有关能量转换的说法正确的是( )
A.煤燃烧是化学能转化为热能的过程
B.化石燃料和植物燃料燃烧时放出的能量均来源于太阳能
C.动物体内葡萄糖被氧化成CO2是热能转变成化学能的过程
D.植物通过光合作用将CO2转化为葡萄糖是太阳能转变成热能的过程
1.(08上海卷)已知:H2(g)+F2(g) 2HF(g)+270kJ,下列说法正确的是( )
2HF(g)+270kJ,下列说法正确的是( )
A.2L氟化氢气体分解成1L的氢气和1L的氟气吸收270kJ热量
B.1mol氢气与1mol氟气反应生成2mol液态氟化氢放出的热量小于270kJ
C.在相同条件下,1mol氢气与1mol氟气的能量总和大于2mol氟化氢气体的能量
D.1个氢气分子与1个氟气分子反应生成2个氟化氢分子放出270kJ
第二节:短文改错: (15分)
Ladies and gentlemen,
May I pay your attention, please? Now we are 76_________ looking for a passenger, Mr Brown, he is from America. 77_________ And he is now leaving Beijing to America by Flight 2748. 78________ But we were told that Mr Brown forgot his passport as 79_________ well as his wallet in Friendship Hotel where he had stayed. 80_________ The manager of the hotel has just telephoned tell us 81_________ about it. He had just sent his secretary to bring 82_________ the passport and the wallet here and she will come sooner. 83_________ Will Mr Brown go to gate of our airport and wait for your 84_________ passport and wallet? She will arrive at in about ten minutes. 85_________ Thank you!
第一节 单词拼写(共10小题;每小题1分,满分10分)
根据下列句子及所给汉语注释,在句子右边的横线上写出空缺出各单词的正确形式。(每空只写一词)
66. The information will need ________(更新) from time to time.
67. His ______(突然) arrival surprised me greatly.
68. On the ______(相反的), I now feel as if that was when my life really began.
69. The fast food restaurant tries to ________(保证、担保)that customers are served quickly.
70. Poetry _________(贡献,投稿) to a higher quality of life.
71. It’s not _________(可靠的) to judge a man only by his looks.
72. People who have _____(目击) an accident often wish that they had done things differently.
73. The little boy has much difficulty ________(观察)the birds’ behavior at night.
74. He bought a second-hand house with a lot of _______(家具) in it.
75. You ________(误解) me. I didn’t mean to hurt you.
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com