
25.“ _____ to Michelle and having two beautiful girls in my house never allows me to look down upon women,” said Obama.
A.Marrying B.Being married C.Having married D.Married
24.People tend to think that children’s IQ is _____ determines how well they are going to do in their future life.
A.what B.that C.which D.where
23.President Hu Jintao urged every effort _____ to rescue and treat the injured when the earthquake happened in Sichuan in 2008.
A.is made B.must be made C.be made D.should make
22.---This is my book.
---_____________ It has my name on it.
A.Here you are! B.How do you find it?
C.It’s hard to say. D.You must be joking!
第一节、单项选择(共15小题,每小题1分,满分15分)
21.Chinese Vice-Premier called on Thursday for _____ all-out effort to ensure _____ food and drug safety.
A.an; a B.an;\ C.the; the D.\;the
32.9g+122.1g-100X/106
X = 21.2g
碳酸钠的质量分数=(21.2g/32.9g)×100%=64.4% - --------1分
--------1分
(2)原混合物固体中氯化钠的质量=(32.9-21.2)g =11.7g
钠元素与氯元素的质量比
=(21.2×46/1062+11.7×23/58.5)g: (11.7×35.5/58.5)g
= (9.2+4.6)g: 7.1g
=13.8g: 7.1g
=138:71 ------------1分
答:原固体混合物中碳酸钠的质量分数为64.4%;原混合物固体中钠元素和氯元素的质量比为138: 71。
35.(3分)
(1)解:设原固体混合物中碳酸钠的质量为X 。
Na2CO3+CaCl2=CaCO3↓+2NaCl
106 100 117

 X
100X
117X
X
100X
117X
106 106

 (32.9-X) g + 117X/106
(32.9-X) g + 117X/106
34.(3分)
|
2H2O2 ==== 2H2O + O2↑……………………….1分
68 32
34g x
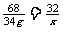 ……………………………………….….1分
……………………………………….….1分

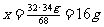 ……………………………………1分
……………………………………1分
答:略
33.(7分)
(1)漏斗
(2) CaCO3
(3)CaO+H2O==Ca(OH)2, Na2CO3+Ca(OH)2=CaCO3↓+2NaOH
K2CO3+ Ca(OH)2=CaCO3↓+2KOH (写对2个化学方程式给1分)
(4)(若假设Ⅱ成立,操作步骤、实验现象、结论对应正确给分)
|
实验步骤 |
实验现象 |
结论和化学方程式 |
|
取滤液分别放在三支试管中, 第一支试管中滴入足量稀盐酸 第二支试管中滴入酚酞溶液; 第三支试管中通入二氧化碳 |
无明显现象 酚酞溶液变红色 滤液出现白色浑浊 |
滤液中无Na2CO3,但显碱性,滤液中有氢氧化钙 CO2+Ca(OH)2=CaCO3↓+ H2O 2HCl+Ca(OH)2=CaCl2+2H2O 假设I成立(或合理) |
[实验反思] NaOH、KOH
32. (6分)
(1)澄清的石灰水变浑浊
二氧化碳与水反应生成碳酸,碳酸使紫色石蕊溶液变红色 三
(2)①B中氢氧化钠滴入后,气球逐渐胀大
C试管内液面逐渐上升
②用软塑料瓶代替烧瓶
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com