
1.(2010全国卷1)下列叙述正确的是
A.Li在氧气中燃烧主要生成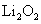
B.将SO2通入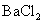 溶液可生成
溶液可生成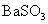 沉淀
沉淀
C.将CO2通入次氯酸钙溶液可生成次氯酸
D.将NH3通入热的CuSO4溶液中能使Cu2+还原成Cu
[解析]A错误,因为Li在空气中燃烧只能生成Li2O,直接取材于第一册课本第二章第三节; B错误,酸性:HCl>H2SO3>H2CO3所以通入后无BaSO3沉淀,因为BaSO3+2HCl=BaCl2+H2O+SO2↑;D错误,溶液中该反应难以发生,先是:2NH3+2H2O+CuSO4=Cu(OH)2↓+(NH4)2SO4,接着Cu(OH)2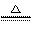 CuO+H20,溶液中NH3不能还原CuO为Cu,要还原必须是干燥的固态!C正确,强酸制弱酸,酸性:H2CO3>HClO,反应为:CO2+H20+Ca(ClO)2=CaCO3↓+2HClO,直接取材于课本第一册第四章第一节;
CuO+H20,溶液中NH3不能还原CuO为Cu,要还原必须是干燥的固态!C正确,强酸制弱酸,酸性:H2CO3>HClO,反应为:CO2+H20+Ca(ClO)2=CaCO3↓+2HClO,直接取材于课本第一册第四章第一节;
[答案]C
[命题意图]考查无机元素及其化合物,如碱金属,氯及其化合物,碳及其化合物,硫及其化合物,氮及其化合物等A、B、C选项直接取材于高一课本,D取材于高二第一章氮族。
[点评]再次印证了以本为本的复习策略,本题四个选项就直接取材于课本,属于简单题,不重视基础,就有可能出错!
12. Before receiving their awards last night, John and the nine other Life Savers attended (v.) a special reception yesterday hosted (host) by the Prime Minister.
11. There is no doubt that John’s quick thinking and the first aid skills he learned at school saved (v.) Ms Slade’s life. It shows that a knowledge (n.) of first aid can make a real difference (n.).
10. He slowed the bleeding by applying pressure (press) to the wounds until the police and ambulance (n.) arrived.
9. It was John’s quick action and knowledge of first aid that saved Ms Slade’s life.
8. She was lying in her front garden bleeding (bleed) very heavily.
7. John was presented with his award at a ceremony which recognized the bravery (brave) of ten people who had saved the life of another.
6. Cover the burned area with a dry, clean bandage (n.) that will not stick to the skin. Hold the bandage in place with tape. Never put butter, oil or ointments on burns as they keep the heat (hot) in the wounds and may cause infection (infect).
5. Do not rub, because / for this may break any blisters and the wound (n.) may get infected (infect).
4. For second degree burns, keep cloths cool by putting (put) them back in the cold water, squeezing (squeeze) them out and placing (place) them on the burnt / burned (burn) area over and over again for about an hour until pain is not so bad.
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com