
8. It’s not ___ good idea to drive for four hours without ___ break.
A. a ; a B. the ; a C. the ; the D. a ; the
7. A small car is big enough for a family of three ____ you need more space for baggage.
A. once B. because C. if D. unless
6. –Do you know Anna’s telephone number? -- ____. As a matter of fact, I don’t know any Anna, either.
A. I think so B. I’m afraid not C. I hope so D. I’d rather not
2. 全国卷(II)
21.A 22.B 23.D 24.C 25.A 26.B 27.D 28.C 29.A 30.B 31.B 32.D 33.C 34.C 35.A
21.-Would you like to join me for a quick lunch before class? - ______, but I promised Nancy to go out with her. A. I’d like to B. I like it. C. I don’t D. I will 22.-What fruit is in season now? -Pears and apples, ______. A. I know B. I think C. I see D. I feel 23. The performance ______ nearly three hours, but few people left the theatre early. A. covered B. reached C. played D. lasted 24. Let’s learn to use the problem we are facing _____ a stepping-stone to future success. A. to B. for C. as D. by 25. The lawyer seldom wears anything other than a suit ______ the season. A. whatever B. wherever C. whenever D. however 26. I like getting up very early in summer. The morning air is so good ______. A. to be breathed B. to breathe C. breathing D. being breathed 27. - Have you known Dr. Jackson for a long time? -Yes, since she ______ the Chinese Society. A. has joined B. joins C. had joined D. joined 28. You’re driving too fast. Can you drive ______? A. more slowly a bit B. slowly a bit more C. a bit more slowly D. slowly more bit 29. The wet weather will continue tomorrow when a cold front ______ to arrive. A. is expected B. is expecting C. expects D. will be expected 30. -Which of the two computer games did you prefer? -Actually I didn’t like ______. A. both of them B. either of them C. none of them D. neither of them 31. -Have you got any idea for the summer vacation? -I don’t mind where we get ______ there’s sun, sea and beach. A. as if B. as long as C. now that D. in order that 32. The weather was ______ cold that I didn’t like to leave my room. A. really B. such C. too D. so 33. The English spoken in the United States is only slightly different from ______ spoken in England. A. which B. what C. that D. the one 34. After studying in a medical college for five years, Jane ______ her job as a doctor in the countryside. A. set out B. took over C. took up D. set up 35. -Sorry, I made a mistake again. - ______. Practice more and you’ll succeed. A. Never mind B. Certainly not C. Not at all D. Don’t mention it
1. 全国卷I
29.(2010重庆卷)(14分)钒(V)及其化合物广泛应用于工业催化、新材料和新能源等领域.
(1)V2O5是接触法制硫酸的催化剂.
①一定条件下, 与空气反映t min后,
与空气反映t min后, 和
和 物质的量浓度分别为a mol/L和b mol/L, 则
物质的量浓度分别为a mol/L和b mol/L, 则 起始物质的量浓度为 mol/L ;生成
起始物质的量浓度为 mol/L ;生成 的化学反应速率为 mol/(L·min) .
的化学反应速率为 mol/(L·min) .
②工业制硫酸,尾气 用_______吸收.
用_______吸收.
(2)全钒液流储能电池是利用不同价态离子对的氧化还原反应来实现化学能和电能相互转化的装置,其原理如题29图所示.

①当左槽溶液逐渐由黄变蓝,其电极反应式为 .
②充电过程中,右槽溶液颜色逐渐由 色变为 色.
③放电过程中氢离子的作用是 和 ;充电时若转移的电子数为3.01 1023个,左槽溶液中n(H+)的变化量为
.
1023个,左槽溶液中n(H+)的变化量为
.
29.答案(14分)
(1)① ;
;
②氨水
(2)①
②绿 紫
③参与正极反应; 通过交换膜定向移动使电流通过溶液;0.5mol
[解析]本题考查以钒为材料的化学原理题,涉及化学反应速率和电化学知识。
(1)
由S守恒可得, 的起始浓度为(a+b)mol/L。
的起始浓度为(a+b)mol/L。 的速率为单位时间内
的速率为单位时间内 浓度的变化,即b/tmol/(L﹒min)。
浓度的变化,即b/tmol/(L﹒min)。 可以用碱性的氨水吸收。
可以用碱性的氨水吸收。
(2)
①左槽中,黄变蓝即为 生成
生成 ,V的化合价从+5降低为+4,得一个电子,0原子减少,从图中知,其中
,V的化合价从+5降低为+4,得一个电子,0原子减少,从图中知,其中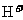 发生了移动,参与反应,由此写成电极反应式。②作为原电池,左槽得电子,而右槽失电子。充电作为电解池处理,有槽中则为得电子,对应化合价降低,即为
发生了移动,参与反应,由此写成电极反应式。②作为原电池,左槽得电子,而右槽失电子。充电作为电解池处理,有槽中则为得电子,对应化合价降低,即为 生成
生成 ,颜色由绿生成紫。③由电极反应式知,
,颜色由绿生成紫。③由电极反应式知,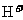 参与了反应。溶液中离子的定向移动可形成电流。n=N/NA=3.01×
参与了反应。溶液中离子的定向移动可形成电流。n=N/NA=3.01× /6.02×
/6.02× =0.5mol。
=0.5mol。
[规律总结]电化学试题的分析一般是从化合价着手,对于原电池,化合价升高的作为负极,化合价降低的作为正极,两极方程式相加即可得总反应。对于电解池,化合价升高作为阳极,降低的作为阴极。两者之间的关系是:正极反应式颠倒即为阳极反应式,负极反应式颠倒即为阴极反应式。
17.(2010江苏卷)(8分)下表列出了3种燃煤烟气脱硫方法的原理。
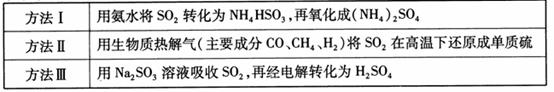
(1) 方法Ⅰ中氨水吸收燃煤烟气中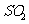 的化学反应为:
的化学反应为:



能提高燃煤烟气中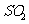 去除率的措施有
▲ (填字母)。
去除率的措施有
▲ (填字母)。
A.增大氨水浓度
B.升高反应温度
C.使燃煤烟气与氨水充分接触
D. 通入空气使 转化为
转化为
采用方法Ⅰ脱硫,并不需要预先除去燃煤烟气中大量的 ,原因是▲(用离子方程式表示)。
,原因是▲(用离子方程式表示)。
(2) 方法Ⅱ重要发生了下列反应:


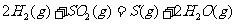





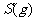 与
与 反应生成
反应生成 的热化学方程式为
。
的热化学方程式为
。
(3) 方法Ⅲ中用惰性电极电解 溶液的装置
溶液的装置
如右图所示。阳极区放出气体的成分为 。
(填化学式)
[答案]
(1)AC

(2)S(g)+O2(g)= S O2(g) H=-574.0kJmol-1
(3) O2 SO2
[解析]本题考察的知识比较散,涉及到环境保护,一道题考察了几个知识点。覆盖面比较多。但盖斯定律、热化学方程式、离子方程式、点击方程式都是重点内容(1)提高SO2的转化率,可以增大氨水的浓度、与氨水充分接触;不需要通入CO2的原因是因为HCO3+SO2=CO2+HSO3而产生CO2 (2)主要考察盖斯定律的灵活运用。适当变形,注意反应热的计算。不要忽视热化学方程式的书写的注意事项。(3)阴极的电极产生的气体为O2和SO2.
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com