
*It could get hot fast
*It should be used with words in an e-mail;
*It might make you 80._________ when getting junk mails.
Section B
Directions: Read the following passage. Answer the questions according to the information given in the passage and required words limit.
Scientists have determined it's not advisable to hurry marriage. But what’s the best age to wed?
When Avril Lavigne announced she was splitting from her husband, comments from her friends suggested that she was only 21 when she tied the knot and later she said that she realized she'd been too young to make such a life-altering decision. Could fellow young celebrity divorcées(离婚者) Reese Witherspoon, Kate Hudson, and Britney Spears have also hit the same age-related issue?
The Magic Number
There are practical reasons for the mid-20s dividing line, and most of them boil down to two biggies: education and money.
It turns out that the more years of higher education a woman has under her belt on her wedding day, the lower the chances that she'll get divorced ... and by 25, you're more likely to have earned a degree or two. Educated women tend to be more confident about who they are and less willing to settle for a man who doesn't meet their standards.
Odds(可能性) are that by 25 you're also supporting yourself, so there's less incentive(刺激; 鼓励)for you to rush into marriage because you're seeking financial security from him. But the marriage-related benefits of working and having money of your own go beyond feeling secure. Learning to budget your cash carefully when you're single will help you avoid financial problems-one of the main causes of couple fights-for the rest of your life.
Knowing the Real You
At 25, you've had time for some crucial life experiences, including a relationship or two that may have improved your Mr. Right radar. You've probably dated enough to have a better idea of what you don't want in a man, which makes it easier to know what you can live with and can't live without.
Perhaps the most important aspect of waiting is that you'll know what your goals and values really are. While you don't want to marry someone just like you, marriage is a lot easier if you two share a similar outlook on life.
Twenty-four and already married to the man of your dreams? Don't worry: Many young marriages survive. But given the choice, you might consider putting off the big day until your mid-20s or later.
81. What main factors influence the mid-20s dividing line? (No more than 3 words)
_______________________________________________________________________________
*74___________: Facemail can let your e-mails read by a “person”;
*Feature: Facemail faces are 75.______________and changeable;
*76.____________: Life FX
*Future of Facemail: ①It is sure to have 77._____________;
②It may be used in a computer screen 78.___________ to help mothers;
* I was greeted by 72.___________ on my computer when I opened my e-mail the other day;
* She kept reading to me an e-mail from my brother about a high-speed Internet connection;
* Sometimes she would stop to smile or 73._______________;
2010.05
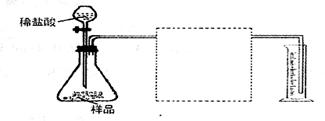
26.(13分)为测定碳酸钙样品(含杂质二氧化硅)的纯度,
某兴趣小组的同学设计了如下几个实验方案:
方案Ⅰ:①称取碳酸钙样品mg②用c1mol·L-1盐酸V1mL(过量)溶解样品③取溶解后的溶液体积的十分之一,用c2mol·L-1NaOH溶液滴定,恰好用去V2mL
方案Ⅱ:①称取碳酸钙样品mg ②高温煅烧直至质量不再改变,冷却后称量,剩余固体质量为m1g.
方案Ⅲ:①称取碳酸钙样品mg②加入c1mol·L-1盐酸V1mL(过量)溶解样品③过滤并取出滤液④在滤液中加入过量c3mol·L-1Na2CO3溶液V3mL⑤将步骤④中的沉淀滤出、洗涤、干燥、称重为m2g。
根据以上实验方案,回答下列问题:
(1)方案I中碳酸钙纯度计算公式为 。
(2)方案Ⅱ中高温锻烧样品应在 (填写仪器名称)中进行;为保证实验的准确性,整个实验过程中至少要称量 次。
(3)方案Ⅲ中不需要的数据是 (填选填编号)。
A.c3、V3 B.c1、V1 C.m2 D.m
(4)方案Ⅲ中为减少实验误差,必要的操作是 (填选项编号)。
A.精确测定Na2CO3溶液中体积为V3mL
B.精确配制Na2CO3溶液浓度为c3mol·L-1
C.将步骤③所得沉淀洗涤,洗液并入④中
(5)方案IV:称取碳酸钙样品mg,按下图装置进行实验,根据实验原理,在图中方框内补充必要的仪器,并注明所用试剂名称。
 温州中学2009学年第二学期期末考试
温州中学2009学年第二学期期末考试
25.(14分)过氧化氢是重要的氧化剂、还原剂,它的水溶液又称为双氧水,常用作消毒、杀菌、漂白等。某化学兴趣小组取一定量的过氧化氢溶液,准确测定了过氧化氢的含量,并探究了过氧化氢的性质。
Ⅰ.测定过氧化氢的含量。请填写下列空白:
(1)移取10.00 mL密度为ρ g·mL-1的过氧化氢溶液至250mL ___________(填仪器名称)中,加水稀释至刻度,摇匀。移取稀释后的过氧化氢溶液25.00mL至锥形瓶中,加入稀硫酸酸化,用蒸馏水稀释,作被测试样。
(2)用高锰酸钾标准溶液滴定被测试样,其反应的离子方程式如下,请将相关物质的化学计量数及化学式填写在方框里。

(3)滴定时,将高锰酸钾标准溶液注入_________(填“酸式”或“碱式”)滴定管中。滴定到达终点的现象是__________________________________________________________。
(4)重复滴定三次,平均耗用c mol·L-1 KMnO4标准溶液V mL,则原过氧化氢溶液中过氧化氢的质量分数为___________________。
(5)若滴定前滴定管尖嘴中有气泡,滴定后气泡消失,则测定结果_________(填“偏高”或“偏低”或“不变”)。
Ⅱ.探究过氧化氢的性质
该化学兴趣小组根据所提供的实验条件设计了两个实验,分别证明了过氧化氢的氧化性和不稳定性。(实验条件:试剂只有过氧化氢溶液、氯水、碘化钾淀粉溶液、饱和硫化氢溶液,实验仪器及用品可自选。)请将他们的实验方法和实验现象填入下表:
|
实 验 内 容 |
实 验 方 法 |
实 验 现 象 |
|
验证氧化性 |
|
|
|
验证不稳定性 |
|
|
24、(4分)阿魏酸在食品、医药等方面有若广泛用途。一种合成阿魏酸的反应可表示为
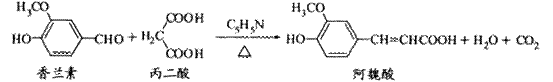
下列说法正确的是
A. 可用酸性KMnO4溶液检测上述反应是否有阿魏酸生成
B. 香兰素、阿魏酸均可与Na2CO3、NaOH溶液反应
C. 通常条件下,香兰素、阿魏酸都能发生取代、加成、消去反应
D. 与香兰素互为同分异构体,分子中有4种不同化学环境的氢,且能发生银镜反应的酚类化合物共有2种
23.(12分)在25℃时,用石墨电极电解2.0 L0.5 mol·L-1CuSO4溶液。5 min后,在一个石墨电极上有6.4g Cu生成。试回答下列问题:
(1)发生氧化反应的是 极,电极反应式为________________________。
(2)若电解后溶液的体积不变,则电解后溶液的pH为____________
(3)若将溶液恢复到与电解前一样,则需加入________mol的___________
(4)若用等质量的两块铜片代替石墨作电极,电解后两铜片的质量相差_______g ,电解液的pH _________。(填“变小”、“变大”或“不变”)
22.(6分)A、B、C、D、E 5瓶透明溶液,分别是HCl、BaCl2、NaHSO4、Na2CO3、AgNO3中的一种。
①A与B反应有气体生成 ②B与C反应有沉淀生成
③C与D反应有沉淀生成 ④D与E反应有沉淀生成
⑤A与E反应有气体生成 ⑥在②和③的反应中生成的沉淀是同一种物质
请填空:
(1)在②和③的反应中,生成的沉淀物质的化学式(分子式)是__________。
(2)D与E反应的离子方程式是____________________________________________
(3)A与E反应的离子方程式是 。
21.(6分)海带中含有丰富的碘元素,某课外兴趣小组设计了下列从海带中提取碘的实验流程:
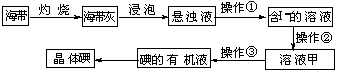
⑴海带灰浸泡过程中常用玻璃棒搅拌的目的是 。
⑵操作①的名称是____________,操作③使用的玻璃仪器有 、 。
⑶操作②是:向含I-的溶液中滴入几滴稀硫酸,再加入一定量质量分数为3%的H2O2。发生反应的离子方程式是______________________________。
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com