
4.2001年11月,美军向躲藏在山洞中的阿富汗恐怖分子使用了一种名为BLU-82的燃料炸弹,这种炸弹爆炸能耗尽山洞中的氧气,使洞中的生物窒息死亡。该炸弹的主装药是环氧乙烷(化学式为C2H4O)。关于环氧乙烷的下列说法正确的是( )
A、它是氧化物 B、它是含有3种元素的混合物
C、它的摩尔质量是44g D、1 mol环氧乙烷含有2NA个碳原子
3.将下列各组物质按单质、氧化物、酸、碱、盐分类顺序排列,其中正确的是( )
A、水银、干冰、硫酸、烧碱、食盐
B、碘酒、冰、盐酸、火碱、食盐
C、氢气、二氧化硫、硝酸、纯碱、胆矾
D、铜、氧化铜、醋酸、石灰水、氯化铜
2.下列各组内物质的转化,只通过一步反应不能完成的是( )
A、Zn→H2 B、KMnO4→MnO2 C、CO2→CaCO3 D、Cu→Cu(OH)2
1.在我们的日常生活中出现了“加碘食盐”、“增铁酱油”、“高钙牛奶”、“富硒茶叶”、“含氟牙膏”等商品。这里的碘、铁、钙、硒、氟,应理解为( )
A、单质 B、分子 C、元素 D、氧化物
I was cleaning out an old box when an old card caught my eye : Queen City Casket Company . “What is it ?” I wondered . I 36 it over . There , in faded ink , was a hand-scrawled(手写的) 37 . Immediately my mind traveled 38 many years .
I was nine years old , walking down the cold , wet streets of Springfield , with a bag of magazines on my shoulder . On my 39 that day , I came to that Company finally , whose owner , Mr Rader , had always taken me there to ask his workers 40 they wanted any magazines .
Shaking off the 41 like a wet dog , I entered Mr Rader’s office . After a quick glance he 42 me over to the fire-place . Noticing the 43 in the top of my 44 , he said , “ Come with me !” pulling me into his pickup 45 . We pulled to a stop before a shoe store . Inside , a salesman 46 me with the finest pair of Oxfords I had 47 seen . I 48 about 10 feet tall when I got up 49 them . “ We’d like a pair of new socks too,” Mr Rader said .
Back in his office , Mr Rader took out a 50 , wrote something on it , and handed it to me . With 51 eyes , I read , “ Do to others as you would have them do to you .” He said affectionately (深情地), “Jimmy , I want you to 52 I love you”.
I said good-bye , and for the first time I 53 a flicker of hope that somehow things would be 54 . With people like Mr Rader in the world , there was hope , kindness and love , and that would always make a 55 .
36.A.read B.thought C.turned D.passed
37.A.address B.list C.message D.information
38.A.forward B.so C.ahead D.back
39.A.return B.rounds C.trip D.arrival
40.A.if only B.how C.whether D.why
41.A.dust B.sweat C.tail D.rain
42.A.led B.followed C.watched D.carried
43.A.hole B.mud C.water D.cover
44.A.magazine B.shoe C.sock D.bag
45.A.truck B.factory C.home D.store
46.A.dressed B.fitted C.showed D.comforted
47.A.ever B.already C.never D.hardly
48.A.appeared B.seemed C.looked D.felt
49.A.for B.with C.on D.in
50.A.pen B.paper C.card D.notebook
51.A.tearful B.unbelievable C.curious D.puzzled
52.A.admit B.know C.consider D.express
53.A.sensed B.received C.lost D.gained
54.A.mistaken B.right C.all right D.possible
55.A.deal B.fortune C.choice D.difference
6、工业制纯碱时,第一步是通过饱和食盐水、氨和二氧化碳反应,获得碳酸

 氢钠结晶,它的反应原理可以用下面方程式表示:NH3+CO2+H2O NH4HCO3,NH4HCO3+NaCl(饱和) NaHCO3↓+NH4Cl
氢钠结晶,它的反应原理可以用下面方程式表示:NH3+CO2+H2O NH4HCO3,NH4HCO3+NaCl(饱和) NaHCO3↓+NH4Cl
以上反应总结果是放热反应。请设计一个实验,用最简单的实验装置模拟实现这一过
程,获得NaHCO3结晶。
可供选择的实验用品有:6mol/L-1盐酸、6mol/L-1的硫酸、浓氨水、氢氧化钠、石灰石、氯化铵、食盐、蒸馏水、冰、以及中学化学常用仪器。
(1)画出实验装置示意图(包括反应时容器中的物质),并在图中玻璃容器旁自左至右分别用A、B、C……符号说明。
说明①本题装置示意图中的仪器可以用下面的方式表示
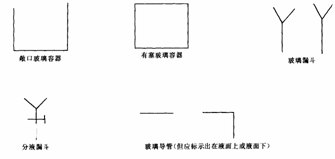 图4-7
图4-7
②铁架台、石棉网、酒精灯、玻璃导管之间的联接胶管等,在示意图中不必画出。如需加热,在加热的仪器下方,标出“△”表示。
(2)请写出在图4-7上用A、B、C……各玻璃容器中盛放物质的化学式或名称。
A: B: C: D:
(3)利用本题所提供的实验用品,如何判断得到的产品是碳酸氢钠的结晶,而不是碳酸氢铵或食盐结晶。
答: 。
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com