
1ЃЎЯТСаИїзщМгЕуЕФзжЃЌЖСвєШЋЖМЯрЭЌЕФвЛЯюЪЧ
AЃЎрСЙжЃЏВ_ФПЁЁЁЁ йШбіЃЏоыУчжњГЄЁЁЁЁ ЕодьЃЏецкаЁЁ ЁЁЮПНхЃЏЩљУћРЧНх
BЃЎРггЁЃЏФЬРвЁЁЁЁ ЛоЦјЃЏЛхШЫВЛОыЁЁЁЁ ЮлЙИЃЏкИТюЁЁЁЁ ЫмСЯЃЏЭЦБОЫндД
CЃЎчЮчПЃЏитКЗЁЁЁЁ РЃРУЃЏЙІПївЛѓёЁЁЁЁ ЕрСПЃЏчшЮлЁЁЁЁ здкМЃЏшђшђШчЩњ
DЃЎеыОФЃЏФкОЮЁЁЁЁ кЉИшЃЏХЛаФСЄбЊЁЁЁЁ ъЁбЇЃЏрЈЦќЁЁЁЁ хдхЦЃЏащгыЮЏЩп
25ЁЂ(1)ИЦдЊЫиЁЁ (2)ДзЁЁ (3)1.2%
24ЁЂ Ђё БћЁЁ Ђђ 57%ЁЁ
Ђѓ (1)CaCl2ЛђCa(OH)2 (2)Й§ТЫЁЁ ШЁМгШыAШмвКЗДгІКѓЃЌЩЯВуЧхвКгкЪдЙмжаЃЌМгШыЬМЫсФЦШмвКЃЌШчЙћВњЩњАзЩЋГСЕэЃЌЫЕУїAШмвКвбОЙ§СПЁЃ
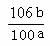 (3)
(3)
23ЁЂ(1)CO32-ЁЁ FeCl3ЁЁ ЂкCa(OH)2+CO2==CaCO3Ё§+H2OЁЁ ЂнFe + CuSO4=Cu+FeSO4
22ЁЂ(1)ЂйЂмЂнЂкЁЁ (2)гаЦјХнВњЩњЁЁ 2Al+6HCl==2AlCl3+3H2Ёќ
21ЁЂHClЁЁ Ca(OH)2ЁЁ CaCl2
20ЁЂ(1)ЂмЁЁ (2)ЂкЁЁ (3)ЂнЁЁ (4)ЂйЁЁ (5)Ђл
19ЁЂNaClЁЁ CO2ЁЁ H2SO4ЁЁ Ca(OH)2ЁЁ H2ЁЁ CuSO4 КЭCa(OH)2
18ЁЂСђЫсЁЁ ЩеМю
17ЁЂ(1)CaCO3 CaO+CO2ЁќЁЁ (2)3Fe+3O2
CaO+CO2ЁќЁЁ (2)3Fe+3O2 Fe3O4
Fe3O4
(3)Zn+H2SO4==ZnSO4+H2ЁќЁЁ (4)NaOH+HCl==NaCl+H2O
(2) (4)
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com