
6. Li Ao insisted that he did not come to see the old China he had lived in, _______ “to see the new China”.
A. but rather B. other than C. rather than D. but also
5. Unable to work at a steady job _______ a terminal illness, he decided to volunteer at the local children center.
A. at the cost of B. as a result of C. in case of D. at the risk of
4. It’s reported that the bridge _______ next year will be very long.
A. being built B. building C. to be built D. built
3. Believe it or not, the Great Wall, which _______ by people, should be protected at once.
A. has been destroyed B. is destroyed C. was destroyed D. is being destroyed
2. Not far from the club _______ two gardens, its owner _________ nearly playing bridge with his children.
A. are; seated B. are; seating C. is; seated D. is; seating
第一节 语法和词汇知识(共20小题;每小题1分,满分20分)
从A、B、C、D四个选项中,选出可以填入空白处的最佳选项,并在答题卡上将该项涂黑。
1. He dressed quickly, _______ hurriedly, grabbed his briefcase and hurried off down the hotel corridor.
A. shave B. shaving C. to shave D. shaved
57.(09上海卷12)NA代表阿伏加德罗常数。下列有关叙述正确的是
A.标准状况下,2.24LH2O含有的分子数等于0.1NA
B.常温下,100mL1mol/LNa2CO3溶液中阴离子总数大于0.1NA
C.分子数为NA的CO、C2H4混合气体体积约为22.4L,质量为28g
D.3.4 gNH3中含N-H键数目为0.2NA

56. 09广东化学6)设nA 代表阿伏加德罗常数(NA )的数值,下列说法正确的是
A. 1 mol 硫酸钾中阴离子所带电荷数为nA
B. 乙烯和环丙烷(C3H6 )组成的28g混合气体中含有3nA 个氢原子
C. 标准状况下,22.4L氯气与足量氢氧化钠溶液反应转移的电子数为nA
D.将0.1mol氯化铁溶于1L水中,所得溶液含有0.1nA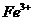
55.(09福建卷8)设NA为阿伏伽德罗常数,下列叙述正确的是
A. 24g镁的原子量最外层电子数为NA
B. 1L0.1mol·L-1乙酸溶液中H+数为0.1NA
C. 1mol甲烷分子所含质子数为10NA
D. 标准状况下,22.4L乙醇的分子数为NA
54.(09广东理科基础20)设nA代表阿伏加德罗常数(NA)的数值,下列说法正确的是
A.22.4 L Cl2中含有nA个C12分子
B.1 L 0.1 mol·L-1 Na2SO4溶液中有0.1 nA个Na+
 C.1 mol H2与1 mol C12反应生成nA个HCl分子
C.1 mol H2与1 mol C12反应生成nA个HCl分子
D.1 mol Ca变成Ca2+时失去的电子数为2nA
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com