
29. ---Could I borrow your dictionary?
---Yes, of course, you____.
A. might B. will C. can D. should
[答案] C
[解析] could表示委婉的语气,并不为时态。答语中of course,表示肯定的语气,允许某人做某事时,用can和 may来表达,不能用could或might。复习: will 与you连用,用来提出要求或下命令。should与you 连用,用来提出劝告。
28. The problem ________ at the meeting next week is of great importance.
A. produced B. being produced
C. to be discussed D. having been produced
[答案]C
[解析] 本题考查非谓语动词作定语的用法。句中有next week,动词不定式则表将来。该句意思为:“下个星期要在会议上讨论的那个问题非常重要。”
27. His first job is in a big bank, from ____ he learnt a lot of information about financial management.
A. where B. what C. that D. which
[答案] D
[解析] 本题考查关系代词的用法。which是指代bank的,后面的从句是说he learnt a lot of information about financial management from the bank.
26. - Haven't seen you for ages. Let's have a get-together next week.
- _________.
A. It's a deal B. It's a long story. C. It's nothing. D. It's only a matter of time.
[答案] A
[解析] 考查习语用法。A. 一言为定。 B. 真是一言难尽。C. 小事情/不足挂齿。 D.这只是时间问题。
25. She didn't _______ it to the airport, so she missed her flight.
A. get B. put C. make D. mean
[答案] C
[解析] 本题考查动词的区别。make it有 “及时到达”的意思。“她没能及时赶到机场,所以错过了航班。”
24. At a rough estimate. Nigeria is ______ Great Britain.
A. three times the size as B. the size three times as
C. three times as the size of D. three times the size of
[答案] D
[解析] 本题考查倍数的用法。倍数应放在比较级或表示尺寸等的名词前。
23. The students are studying Unit 3 this week, and this time next week they _______ Unit 4.
A. are studying B. are to study C. will study D. will be studying
[答案] D
[解析] 本题时态的用法。句后”this time next week”是将来进行时的标志。
22. There exists such a phenomenon that some people waste food __________ others haven't enough.
A. while B. until C. when D. before
[答案] A
[解析] 考查连词的用法。while在这里表示对比转折,有“然而”的意思。
21. Language is _______ systematic means of communicating ideas or feelings by ___ use of conventionalized signs, sounds, gestures, or marks having understood meanings.
A. a; an B. a; a C. a; the D. the; the
[答案] C
[解析] 本题考查冠词的用法。means是单数名词,后一个为具体运用。
16、已知有机物中一个碳原子上连有两个羟基时,易脱水形成碳氧双键;物质A→F有如下转化关系:
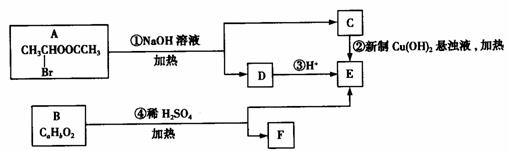
请回答下列问题:
(1)E中含有的官能团的名称是_____________,C跟新制,的氢氧化铜悬浊液反应的化学方程式为:____________________________
(2)已知B的相对分子质量为162,其燃烧产物中n(CO2):n(H2O)=2:1。则B的分子式为_________________
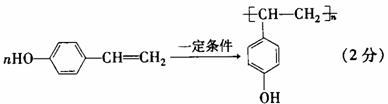
(3)F具有如下特点:①能跟FeCl3溶液发生显色反应;②能发生加聚反应。若F的苯环上的一氯代物只有两种,则F在一定条件下发生加聚反应的化学方程式为__________________________。
(4)化合物G是F的同分异构体,它属于芳香族化合物,能发生银镜反应。则C可能具有______________________种结构,写出其中两种的结构简式_______________________。
解析:(1)由图示及已知信息可得反应①为:
CH3CHClOOCCH3+2NaOH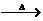 CH3CHO+CH+COONa+H2O
+NaBr。所以物质C是CH3CHO、D是CH3COONa、E是CH3COOH;故E中含有的官能团是羧基,C跟新制的氢氧化铜悬浊液反应的化学方程式为:CH3CHO+2Cu(OH)2
CH3CHO+CH+COONa+H2O
+NaBr。所以物质C是CH3CHO、D是CH3COONa、E是CH3COOH;故E中含有的官能团是羧基,C跟新制的氢氧化铜悬浊液反应的化学方程式为:CH3CHO+2Cu(OH)2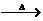 CH3COOH+Cu2O↓+2H2O。
CH3COOH+Cu2O↓+2H2O。
(2)对于B物质,其分子式为CaHbO2;由其相对分子质量为162,燃烧产物中n(CO2) :n(H2O)=2:1,可确定a=b,162-32=130,即(CH)n的式量为130,所以n=10,故B的分子式是C10H10O2.
(3)依据F具有的特点并结合B在稀H2SO4 、加热条件下生成乙酸和F,可判断B为乙酸酯类,进而确定F的结构简式为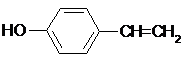 (4)化合物G是F的同分异构体,它属于芳香族化合物,能发生银镜反应。则G中含有醛基和苯环,即可推出其所有结构。
(4)化合物G是F的同分异构体,它属于芳香族化合物,能发生银镜反应。则G中含有醛基和苯环,即可推出其所有结构。
答案:(1)羧基(1分) CH3CHO+2Cu(OH)2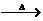 CH3COOH+Cu2O↓+2H2O(2分)
CH3COOH+Cu2O↓+2H2O(2分)
(2)C10H10O2(3分)
(3) 
(4)4 (2分) 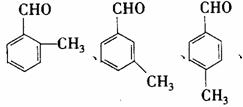 (2分)
(2分)
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com