
5.Your plan looks good. I hope it will really work. It’s time to implement it and see if it’s as brilliant as you claim.
a. instrument b. take apart c. change d. carry out
4.We have rather lofty expectations for you, son. You will attend college. You will become rich and famous. You will be elected president of the United States before you turn forty.
Which word is a synonym of “lofty”?
a. high b. shaky c. small d. lowly
3.Kathy was looking for a strong but light material to use for making her water jugs. Unfortunately, she chose noodelite. It proved to too porous to hold jelly.
A porous material _____.
a. is good for holding things that you pour b. protects you in pouring rain c. allows liquids to flow through it d. is necessary for making bowling balls
2.Only an hour or so had passed before a tremendous roar shook the ground. At that very moment, a strange grey creature materialized before our eyes. It resembled a lizard in shape. It was about ten feet high at the shoulders and at least fifty feet long.
What did the creature do?
a. It whipped its tail back and forth. b. It stamped its feet. c. It showed its sharp teeth. d. It appeared.
1.We walked slowly down the trail with great trepidation. No one who had gone this way had ever been heard from again. Had they simply found a better place to settle on this dark planet? We doubted that.
Which word is a synonym of “trepidation”?
a. movement b. worry c. enjoyment d. laughter
31.D 32. A 33.B 34.A 35. A 36.A 37. B 38. A 39.C 40.D
Use the context to help you choose the best meaning or synonym for each highlighted word.
21. B 22. D 23. A 24.B 25.D 26.D 27. D 28. C 29. D 30. D
11. A 12. C 13. A 14. B 15. C 16. A 17. D 18. C l9. D 20. B
1. B 2. A 3. B 4. B 5. C 6. C 7. D 8. B 9. A 10. D
20.(12分)本题包括A、B两小题,分别对应于“物质结构与性质”和“实验化学”两个选修模块的内容。请选择其中一题作答。若两题都做,则按A题评分。
A.CuCl和CuCl2都是重要的化工原料,常用作催化剂、颜料、防腐剂和消毒剂等。已知:
①CuCl可以由CuCl2用适当的还原剂如S02、SnCl2等还原制得:
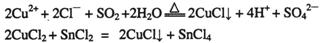
②CuCl2溶液与乙二胺(H2N-CH2-CH2-NH2)可形成配离子:

请回答下列问题:
(1)基态Cu原子的核外电子排布式为_________。H、N、O三种元素的电负性由大到小的顺序是_____。
(2)SO2分子的空间构型为____________。与SnCl4互为等电子体的一种离子的化学式为_________。
(3)乙二胺分子中氮原子轨道的杂化类型为_____________。乙二胺和三甲胺[N(CH3)3]均属于胺,但乙二胺比三甲胺的沸点高的多,原因是_____________________________________________。
 (4)②中所形成的配离子中含有的化学键类型有__________。(填字母)
(4)②中所形成的配离子中含有的化学键类型有__________。(填字母)
a.配位键 b.极性键 c.离子键 d.非极性键
(5)CuCl的晶胞结构如右图所示,其中Cl原子的配位数为_________。
B.阿司匹林(乙酰水杨酸)是常用的解热镇痛药,以下是合成阿司匹林的工艺流程。
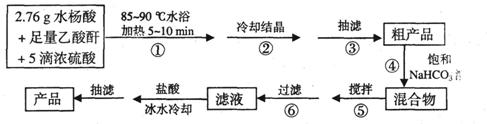
已知:①反应方程式为
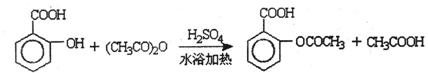
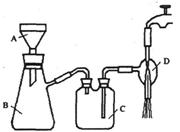
②抽滤的实验装置如右图所示。
请回答有关问题:
(1)仪器A的名称是_____________。
(2)在步骤②中,若冷却结晶时未出现结晶,可以___________,
促使晶体析出。
(3)实验时,当仪器B中液面高度快达到支管口位置时,应进行
的操作是____________________________________________。
(4)仪器C中两玻璃导管的位置是否正确?答:_________。(填“正确”或“不正确”)
(5)在步骤④中,用饱和NaHC03溶液可以将阿司匹林和剐产物等分离,其化学原理是
_________________________________________。要检测产品中是否含有水杨酸,其实验操作是_____________________________________________________。
(6)在步骤⑤中,搅拌使反应充分进行至_____________为止。
(7)若实验得到2.70g纯净的阿司匹林,则产品的产率为____________。
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com