
23. ------ Do you know Jim quarreled with his brother?
------ I don’t know,________.
A. nor don’t I care B. nor do I care C. I don’t care neither D.I don’t care also
第一节:单项选择(共15题;每小题1分,满分15分)
21----Watch!
----I___but I ____anything unsual.
A.watched, saw B.have watched,see
C.am watching,am not seeing D.am watching,don’t see
22.-- I m not feeling well today.
--Why not _____ some medicine?
A.take B.to take C.taking D.you take
第一节(共5小题。听下面5段对话。每段对话后有一小题,从题中所给的A、B、C三个选项中选出最佳选项。每段对话仅一遍。)
听第一段话,回答第1题。
1.What’s the man looking for?
A.A few stores B.A drug store C.A clinic
听第二段话,回答第2题。
2.Where will the woman go first?
A.To the school B.To a friend’s house C.To the post office
听第三段话,回答第3题。
3.What does the man suggest the woman do?
A.Save time by using a computer B.Buy her own computer C. Borrow Martha’s computer
听第四段话,回答第4题。
4.Why does the woman say that she could not attend the party?
A. She was afraid she might be kept too late
B.She would have something more important to do
C.she was not in the mood(心情) to attend the party
听第五段话,回答第5题。
5.What has the boss done?
A.Rearranged(重新布置) his office B.Cut the woman’s work hours C.Let the woman off for the weekend.
二节(共15小题。听下面几段对话或独白。每段对话后有几个小题,从题中所给的A、B、C三个选项中选出最佳选项。每段对话读两遍。)
听第六段话,回答第6-8题。
6.What is the woman doing in the shop?
A.She is here to buy a bicycle B.She is looking for a job C. She is helping her father
7.Why did the man come to the shop?
A. To look for a new racing bike B. To get a bike to ride to work C. To join a bicycle club
8.What do we know about the man?
A. He is active B. He is warm-hearted C. He is lazy
听第七段话,回答第9-11题。
9.Where does the conversation(谈话) most probably(可能) take place?
A. At the airport B. In the restaurant C. In the office
10.How does the man usually go to work/
A. By bus B. By car C. On foot
11.What do we know about the two speakers?
A.They will have their lunch in a restaurant B.They are journalists(新闻工作者) in the same company C.They will have an interview(面试) for a new job
听第8段话,回答第12-14题。
12.What are the two speakers mainly talking about?
A.the capital(首都) of Sweden B.The woman’ s life C.The woman’s husband
13.Which of the following is wrong according to the dialogue?
A.The woman doesn’t like cowboys B.The woman can milk a cow C.The woman can ride a horse
14.What can we learn from the dialogue?
A.The woman’s husband is a forgetful(健忘的) man B.The woman doesn’t like her hometown
C.The woman’s children enjoy living in the United States
听第9段话,回答第15-17题。
15.What did the woman try when she was looking for a place to rent(租用)
A.Going online B.The housing broker(中间人)company C. The classified ads(分类广告)
16.Why does the woman want to leave her current(当前的) place?
A.She can’t get on well with her roommate B.she will get married C. her roommate’s fiancé(未婚夫) will move in
17.How much will the broker of New York Reality charge(收费) the woman?
A. A Fee(费用) equal to one month’s rent B.A fee equal to one month’s salary(工资) of the woman C.Uncertain(不确定)
听第10段话,回答第18-20题。
18.What’s the purpose(目的) of getting a hot,damp(潮湿的) cloth before dinner?
A.To clean the table B.To put it on the knees(膝部) C. To clean face and hands
19.What do Chinese usually use for dinner?
A.Forks B.Spoons C.Chopstics
20.What do Chinese prefer (更喜欢) to drink at a dinner party?
A.Milk-tea B.Bee C. Coffee
34.(4分)已知反应:3Cl2+8NH3=N2+6NH4Cl
若71gCl2参加反应被氧化的NH3的质量为多少?还原产物的质量为多少?(11.3g 107g)
△
33.(6分)已知铜与浓硫酸的反应为 Cu +2H2SO4(浓) = CuSO4+SO2↑+2H2O,当反应中生成32克SO2气体时,消耗H2SO4的质量是多少克?其中有多少克H2SO4作为氧化剂被还原?
(98克,49克)
32.某溶液中含有X-、Y2-、Z2-三种常见的无机离子。如下图框图所示,发生了一系列化学反应。第④步反应生成的白色沉淀中含Y2-.

|
|
|||||
|
||||||
|
|
⑴判断X-、Y2-、Z2-分别为________、________、________(写离子符号)。
⑵写出①、②、④、⑤步反应的离子方程式。
、⑴Cl-、SO42-、CO32- (每空1分) ⑵①SO42-+Ba2+==BaSO4↓ CO32-+Ba2+==BaCO3↓
②Ag++Cl-==AgCl↓ ④BaCO3+2H+==Ba2++CO2↑+H2O
⑤Ca2++2OH-+CO2==CaCO3↓+H2O (每个离子方程式2分)
30.除去括号中杂质,写出所加试剂与反应的离子方程式。
⑴ Cl-(SO42-),所加试剂:BaCl2 _________,离子方程式:___________________________。
⑵CO2(HCl),所加试剂:NaHCO3 _________,离子方程式:___________________________。
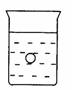 31.在一烧杯中盛有稀H2SO4溶液,同时有一表面光滑的塑料小球悬浮
31.在一烧杯中盛有稀H2SO4溶液,同时有一表面光滑的塑料小球悬浮
于溶液中央(如图所示)。向该烧杯里缓缓注入与稀H2SO4等密度的
Ba(OH)2溶液至恰好完全反应。在此实验过程中
⑴烧杯里观察到的实验现象有:
①____________________________________________,
②____________________________________________。
⑵写出实验过程中反应的离子方程式________________________________________。
⑴①产生白色沉淀 ②小球下沉 ⑵Ba2++2OH-+2H++SO42-==BaSO4↓+2H2O (离子方程式2分)
29.请根据反应①X2+2Z-=2X-+Z2 ②Z2+2W-=2Z-+W2 ③W2+2Y-=2W-+Y2 判断: (1)哪些物质具有氧化性,并指出氧化能力强弱顺序。 ___________ _____________ X2>Z2>W2>Y2_____________________________________________ (2)反应W2+2X-=2W-+X2能否进行,说明理由。 _____________ _W2不能将X-氧化___________________________________ ________________
28.(4分)现有以下物质:①KCl晶体 ②液态HCl ③醋酸 ④汞 ⑤CaCO3固体
⑥稀硫酸 ⑦酒精(C2H5OH) ⑧液态KNO3,⑨液态SO3请回答下列问题(用序号):
⑴以上物质中能导电的是_④⑥⑧________________。
⑵以上物质中属于电解质的是_____①②③⑤⑧______________。
⑶以上物质中属于非电解质的是____⑦ ⑨________________。
⑷以上物质中溶于水后形成的水溶液能导电的是_①②③⑥⑧⑨ ___________________。
27.请把符合要求的化学反应方程式的字母编号填入横线上: (1)既属于分解反应又是氧化还原反应的是______ D ____ 。 (2)属于化合反应,但不是氧化还原反应的是________ E ________ 。 (3)既属于化合反应,又是氧化还原反应的是________ B ______ 。 (4)属于分解反应,但不是氧化还原反应的是_________ A _______ 。 (5)不属于四种基本反应类型的氧化还原反应的是______ F ______ 。 A.2NaHCO3 == Na2CO3 +CO2↑+H2O B.2Na+Cl2 == 2 NaCl C.Zn+CuSO4 =Cu+ZnSO4 D.2KMnO4== K2MnO4+MnO2+O2↑ E.CaO+CO2=CaCO3 F.4FeS2+11O2== 2Fe2O3+8SO2
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com