
3. Tired after the long journey, I still enjoyed meeting the aliens on the space station
2. Hit by the lack of fresh air, he got a bad headache,
1. Frightened by the loud noise, I went to see what was happening.
3. Confused by the new surroundings, I was hit by the lack of fresh air
When I was confused by the new surrounding, I was…
Arriving home, he showed me into a large bright, clean room.
When he is arriving home, he showed me into…
Exhausted, I slid into bed and fell fast asleep
As I was exhausted, I slid into bed and fell fast asleep.
过去分词作状语,意义上相当于状语从句, 表示时间, 条件,原因, 伴随状况等
Whenever praised, he blushed
United, we stand, divided, we fall
Written in a hurry, the book is full of errors
Although born in Germany, John lives and works in U.S.A
PAGE 20, EX 2
4.He spoke loudly in order to make himself ____ (hear)
Sentence patterns
Worried about the journey, I was unsettled for the first few days
As I was worried about the journey, I was unsettled for the first few days
Well known for their expertise, his parents’ company named “future tours” transported me safely into the future in a time capsule.
His parents’ company was well known for their expertise …
3. There is something wrong with my bike and I have to get it __________(repair).
2. The girl ________(write) a letter is my cousin
2. Group work
Teaching Procedures:
Step I Dictation
Step II. Grammar
Grammar past participle used as adverbial and attribute
Complete the following sentences with the words given, using their proper forms.
1, I like reading the novels______ (write) by him
1. Inductive Method
12.(2010年枣庄模拟)二氧化硫是常用的化工原料,也是大气污染物。硫酸生产中,SO2催化氧化生成SO3:2SO2+O2  2SO3。
2SO3。
(1)若反应在恒温、恒容下进行,判断该反应达到平衡状态的标志是____________(填序号)。
A.SO2和SO3的浓度相等
B.容器内压强保持不变
C.SO2和SO3生成速度相
D.SO2百分含量保持不变
(2)SO2的平衡转化率(a)与温度(T)和压强(p)关系如下图所示。
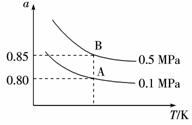
①图象中平衡常数K(A)____________K(B)(填“大于”或“等于”或“小于”),反应的ΔH____________0(填“大于”或“等于”或“小于”)。
②图中虚线的温度下,将2 mol SO2和1 mol O2置于10 L密闭容器中,达平衡后,体系的总压强为0.10 MPa,反应的平衡常数是____________。
(3)用NaOH溶液吸收SO2气体可生成正盐或酸式盐。在25 ℃下,某浓度的NaHSO23溶液的pH=6;则溶液中c(H2SO3)____________c(SO32-)(填“大于”或“等于”或“小于”);若忽略水的电离及H2SO3的二级电离,已知H2SO3[FYKN]H++HSO32-常数K1=1.23×10-2,则0.1 mol·-1L H2SO3的H+的物质的量浓度c(H+)=____________
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com