
in one’s opinion, that is to say, for example, for instance, as a matter of
fact, in fact, namely
A. As a matter of fact, advertisement plays an informative
role in our daily life.
B. There is one more topic to discuss, namely/that is (
to say ), the question of education.
worse still, moreover, furthermore; but for, in addition, to make matters worse
A. The house is too small for a family of four, and furthermore/besides/what’s
more/moreover /in addition/worse still , it is in a bad location.
on one hand ,on the other hand, on the contrary/contrary to ..., though, for one thing ;for another, nevertheless
A. I know the Internet can only be used at home or in the office, but on the other hand, it is becoming more and more popular for much information as well as clear and vivid pictures.
B. It is hard work; I enjoy it though.
C. Contrary to what I had originally thought, the trip turned out to be fun.
as well as, not only…but (also), including,
A. Not only do computers play an important part in science and technology, but
also play an informative role in our daily life.
B. All of us, including the teachers / the teachers included, will attend the
lecture.
C. He speaks French as well as English.=He speaks English, and French as
well.=He speaks not only English but also French.
D. E-mail, as well as telephones, is playing an important part in daily communication.
--开门见山法。也就是说, 直截了当地提出你对这个问题的看法或要求,点出文章的中心思
想。
1.议论论文:
A. Just as every coin has two sides, cars have both advantages and
disadvantages.
B. Compared to/ In comparison with letters, e-mails are more convenient.
C. When it comes to computers, some people think they have brought us a lot of
convenience. However,...
D. Opinions are divided on the advantages and disadvantages of living in the
city and in the countryside.
E. As is known to all/ As we all know, computers have played an important
role/part in our daily life.F. Why do you go to university? Different people
have different points of view.
2. 书信:A. I am writing to you to apply for admission to your university as a
visiting scholar.
B. I read an advertisement in today’s China Daily and I apply for the job...
C. Thank you for your letter of May 5.
D. How happy I am to receive your letter of January 9.
E. How nice to hear from you again.
3. 口头通知或介绍情况:
A. Ladies and gentlemen, May I have your attention, please. I have an
announcement to make.
B. Attention, please. I have something important to tell you.
C. Mr. Green, Welcome to our school. To begin with, let me introduce Mr. Wang to
you.
4. 演讲稿:
A. Ladies and gentlemen, I feel very much honored to have a chance here to make
a speech on the subject -- A Balance Diet and Health.
B. Good morning everyone! Allow me, first of all, on behalf of all present here,
to extend our warm welcome and cordial greeting to our distinguished guest.
(三)卤素单质的化学性质(相似性及递变性)
由于最外层均为 个电子,极易 电子,因此卤素都是 剂,在自然界均只以 态存在.但随着电子层数递增,原子半径渐 ,核对外层电子的引力渐 ,得电子能力渐 ,其氧化性逐渐 ,主要表现:
。
试题枚举
[例1]下列有关氯的叙述中正确的是
A.液氯和氯水是同一物质
B.红磷在氯气中燃烧产生白色烟雾
C.氯气与水的反应中氯是氧化剂,水是还原剂
D.用氯制漂白粉是为了得到易贮存的漂白剂
解析:液氯、氯水都是液体,但前者是纯氯,后者是氯与水的混合物,时间延长氯水中还逐渐生成盐酸和次氯酸,氯分子渐少,氯离子渐多。如果再有光照, 因分解也在渐少,出现氧气和更多盐酸。氯与水的反应是氯分子中氯原子间发生的自身氧化还原,即歧化反应, 水虽然是反应物,却未参加氧化还原。A、C不正确。
烟是分散在气体中的固体小颗粒,雾是分散在气体里的液体小珠滴。磷在氯气里燃烧的产物三氯化磷是无色液体,可发雾;五氯化磷是浅黄色固体,能形成烟。漂白粉和氯都可以作为漂白剂、消毒剂,且氯更有效。但氯气难贮存,又有剧毒,一般医用、家用的漂白剂和消毒剂使氯气是不合宜的,所以制成漂白粉便于贮存和使用。漂白粉保存得当,如密闭,以防止吸水和 而变质,可以存放较长时间。B、D正确。
答案:B、D。
[例2] 潮湿的氯气、新制的氯水、次氯酸钠及漂白粉的水溶液均能使有色布条褪色,原因是它们均含有 ( )
A. 氯气 B. 次氯酸 C. 次氯酸根 D. 氯化氢
解析 NaClO及Ca(ClO)2的水溶液中,ClO-水解产生HClO无HCl;有色布条的褪色是因HClO的氧化所致。
答案: B
[变式] 用滴管将新制的氯水慢慢滴入盛酚酞的氢氧化钠稀溶液中,当滴到最后一滴时红色突然褪去,发生这一现象的原因可能有两个:
①是由于 (用简要文字说明)
②是由于 (用简要文字说明)
简述用实验方法证明褪色的原因是①还是②
。
[例3]如何鉴别NaCl、NaBr、KI三种白色固体?
解答:
方法一:可用氯水鉴别。
把这三种物质各取少量制成溶液,加入新制的氯水和汽油(或四氯化碳),振荡,分层,使汽油层呈无色的是NaCl;使汽油层呈橙色的是NaBr;呈紫色的是KI。
方法二:可先用焰色反应将KI区分出来。然后再将另外两种物质各取少量制成溶液,加入AgNO3溶液,有白色沉淀生成的是NaCl,有浅黄色沉淀生成的是NaBr。
方法三:直接加入AgNO3溶液。
分别取少量固体制成溶液,分别加入AgNO3溶液,有白色沉淀生成的是NaCl,有浅黄色沉淀生成的是NaBr,有黄色沉淀生成的是KI。
[例4] 多原子分子氰(CN)2、硫氰(SCN)2和(OCN)2的性质与卤素单质相似,故称它们为拟卤素。它们可以生成酸和盐(见下表,表中X代表F、Cl、Br或I)。
|
|
卤素 |
氰 |
硫氰 |
① |
|
“单质” |
X2 |
(CN)2 |
(SCN)2 |
(OCN)2 |
|
酸 |
HX |
HCN |
② |
HOCN |
|
盐 |
KX |
KCN |
KSCN |
③ |
(1)在表中①②③空格处应分别填写_______、_______、_______。
(2)完成下列反应的化学方程式或离子方程式。
①(CN)2和KOH溶液反应的化学方程式为:______________。
②已知阴离子的还原性强弱为:Cl-<Br-<CN-<SCN-<I-。试写出在NaBr和KSCN的混合溶液中加入(CN)2反应的离子方程式:____________________________________。
解析:此题主要考查“运用已学知识进行类推思维的能力”。解题时可用熟知的Cl2、HCl、KCl等物质作参照物。由还原性Br-<CN-<SCN-,可推知氧化性Br2>(CN)2>(SCN)2,因而可判断(CN)2只能与KSCN反应,不能与NaBr反应,亦即由物质的氧化性和还原性的强弱,可以判断氧化还原反应能否发生。
答案:(1)氧氰 HSCN KOCN
(2)①(CN)2+2KOH====KCN+KOCN+H2O
②(CN)2+2SCN-====2CN-+(SCN)2
该题为信息给予题,给予的信息是:拟卤素(如(CN)2)的性质与卤素单质相似,由此而可依据卤素单质的性质来进行类比、模拟来解答习题。这是解答信息给予题的一种方法。
[变式] 溴化碘(IBr)的化学性质与卤素单质相似,能与大多数金属反应生成金属卤化物,和某些非金属单质反应生成相应的卤化物,跟水反应的方程式IBr+H2O=HBr+HIO,下列有关IBr的叙述中,不正确的是 ( )
A. IBr是双原子分子
B.在很多反应中IBr是强氧化剂
C.和NaOH溶液反应生成NaBr和NaIO
D.和水反应时,既是氧化剂又是还原剂
 [例5]在右图的四条直线分别表示钠、镁、铝、铁与足量Cl2反应时,消耗金属的质量(纵轴)与反应掉的氯气质量(横轴)的关系,其中代表铁与Cl2反应的直线是 ( )
[例5]在右图的四条直线分别表示钠、镁、铝、铁与足量Cl2反应时,消耗金属的质量(纵轴)与反应掉的氯气质量(横轴)的关系,其中代表铁与Cl2反应的直线是 ( )
A. a B. b C. c D. d
解析 Cl2与四种金属反应的均为氧化还原反应,在横坐标上取一点作一条垂直于横坐标的直线,分别与a、b、c、d四条直线的交点即为四种金属与等质量Cl2反应时所需的质量。假设这一点的质量为35.5g,此时金属均转移1mol电子,则消耗四种金属的质量分别为:WNa=23g、WMg=12g、WAl=9g、WFe=18.7g,即等质量氯气消耗四种金属的质量是WNa>WFe>WMg>WAl,故a、b、c、d分别代表Al、Mg、Fe、Na与氯气的反应。
答案:C
(二)单质的物理性质
随卤素核电荷数增加,其原子结构的递变而使卤素单质的物理性质呈规律性变化.
归纳:从F2 →I2
1、颜色逐渐 ,状态从 → → ,密度从 → ,熔沸点由 → (原因是 。
2、单质的溶解性--除氟外(与水剧烈反应)在水中溶解度都较 ,且由 → ,都易溶于有机溶剂,下表列出Cl2、Br2、I2在不同溶剂中的颜色.
|
|
水 |
CCl4 |
|
|
Cl2 |
|
|
|
|
Br2 |
|
|
|
|
I2 |
|
|
|
(一)卤素的原子结构
共同点:原子的最外层均为 个电子,都易 1个电子而表现 性;
不同点:核电荷数逐渐 ;电子层数逐渐 ;原子半径依次 ,得电子能力逐渐 ;单质氧化性逐渐 。
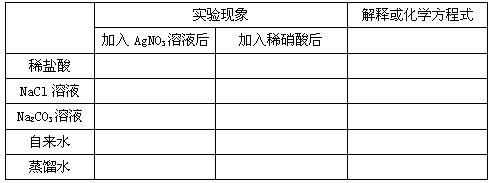
2、原理:被检液中滴入少量 酸化,再滴入 溶液,若产生 ,则可断定溶液中含有氯离子。不可用盐酸酸化,酸化目的是防止( 、 、 、 )影响。
+ AgNO3(aq) → + 稀硝酸 →白色↓
解释:Ag+ + Cl- = AgCl↓
2Ag+ + 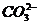 = Ag2CO3↓ Ag2CO3 + 2H+ = 2Ag+ + H2O + CO2↑
= Ag2CO3↓ Ag2CO3 + 2H+ = 2Ag+ + H2O + CO2↑
1、填写下列表格:
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com