
“It’s perfect for 39 ,” my mother continued. “I 40 someone had made a dress like this for me.”
41 you can’t wear it, I thought. But I knew Mom had spent a lot of time on the dress. Only the world’s most 42 daughter would refuse. reluctantly(不情愿的). I put it on.
All through church I prayed, Lord, let me get out of here __43 anyone seeing me. Especially Dennis Pearce, the boy I had a crush on. He was one of the cutest (bright) guys at Neptune High. Although we were in some of the same classes, Dennis had 44 taken any notice of me.
At the end of the service I 45 for the door. But I had to wait on the 46 while my parents chatted with their friends. Just a little while longer… Then 47 the corner of my eye I saw the Peace approaching. 48 I could escape, Dennis was right beside me.
I started gabbing a mile a minute, hoping if I kept it 49 he wouldn’t notice my horrible dress. “I am going to college in September,” I said.
“That’s great,” Dennis replied. “I got 50 to the police academy.”
“Wow!” I said. Somehow I kept the conversation going. Soon we were walking to the parking lot together. The next thing I knew Dennis had first asked me 51 .
We courted through college, and eventually got married. Months after our wedding I asked Dennis if he remembered the day 52 he had first asked me out.
“_ 53 bet I do,” he said. “You were always 54 in school, almost standoffish (coldhearted). I didn’t think you’d be much fun. But you were so animated (lively) when we talked on the church steps, I wanted to get to know you better.” Maybe that 55 yellow dress wasn’t what I would have chosen, but that day it was the perfect dress for me.
36. A. Strange B. Surprise C. Interesting D. Puzzled
8.(9分)在一定温度下,将8 molSO2和4 molO2放入密闭容器内进行反应:
2SO2(g)
+ O2(g)  2SO3(g)
2SO3(g)
(1)如果原容器内的压强为3×106 Pa,平衡时SO2的转化率为80%,求平衡时容器内的压强.
(2)如果平衡时混合气体中SO2所占的体积百分数为60%,求SO2平衡时的物质的量.
7.(12分)在一固定容积的密闭容器中,保持一定条件下进行以下反应:X(气)+2Y(气) 3Z(气),已知加入1
molX和3 molY。达到平衡后,生成a mol Z。
3Z(气),已知加入1
molX和3 molY。达到平衡后,生成a mol Z。
(1)在相同实验条件下,若在同一容器中改为加入2 molX和6 molY,达到平衡后,Z的物质的量为 。
 (2)在相同实验条件下,若在同一容器中改为加入2 molX和8 molY,若要求在反应混合气体中Z体积分数不变,则还需加入Z的物质的量为
。
(2)在相同实验条件下,若在同一容器中改为加入2 molX和8 molY,若要求在反应混合气体中Z体积分数不变,则还需加入Z的物质的量为
。
(3)在相同实验条件下,若在同一容器中改为加入0.5molX,则需加入 mol Y,
 mol Z,才能使平衡时Z为0.9 a mol。
mol Z,才能使平衡时Z为0.9 a mol。
6.工业上制备纯硅反应的热化学方程式如下:SiCl4(g)+2H2(g) Si(s)+4HCl(g);△H=+QkJ/mol(Q>0)。某温度、压强下,将一定量反应物通入密闭容器进行以上反应(此条件下为可逆反应),下列叙述正确的是
Si(s)+4HCl(g);△H=+QkJ/mol(Q>0)。某温度、压强下,将一定量反应物通入密闭容器进行以上反应(此条件下为可逆反应),下列叙述正确的是
A.反应过程中,若增大压强能提高SiCl4的转化率
B.若反应开始时SiCl4为1mol,则达平衡时,吸收热量为QkJ
C.反应至4min时,若HCl浓度为0.12mol/L,则H2的反应速率为0.03mol/(L·min)
D.当反应吸收热量为0.025QkJ时,生成的HCl通入100mL 1mol/L的NaOH溶液恰好反应
5.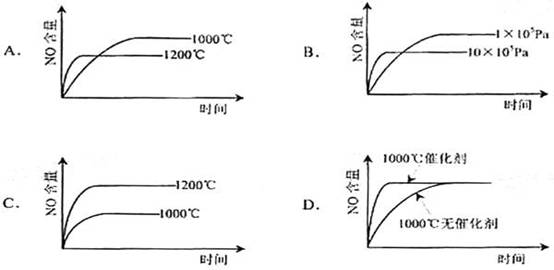 已知:4NH3(g)+5O2(g)=4NO(g)+6H2O(g).
△H= -1025KJ/mol该反应是一个可逆反应。若反应物起始物质的量相同,下列关于该反应的示意图不正确的是
已知:4NH3(g)+5O2(g)=4NO(g)+6H2O(g).
△H= -1025KJ/mol该反应是一个可逆反应。若反应物起始物质的量相同,下列关于该反应的示意图不正确的是
4. 将固体NH4I置于密闭容器中,在一定温度下发生下列反应:
① NH4I(s)= NH3(g)+HI(g) ②2HI(g) =H2(g)+I2(g)
达到平衡时,c(H2)=0.5mol·L-1,c(HI)=4mol·L-1,则此温度下反应 ①的平衡常数为
A.9 B.16 C.20 D.25
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com