
6. [A] in [B] by [C] after [D] over
5. [A] discriminating[B] distinguishing [C] determining [D] diminishing
4. [A] innate [B] intact [C] integral [D] integrated
3. [A] panel [B] crew [C] band [D] flock
2. [A] agency [B] organization [C] institution [D] authority
As former colonists of Great Britain, the Founding Fathers of the United States adopted much of the legal system of Great Britain. We have a “common law”, or law made by courts__1__a monarch or other central governmental__2__like a legislature. The jury, a__3__of ordinary citizens chosen to decide a case, is an__4__ part of our common-law system.
Use of juries to decide cases is a__5__feature of the American legal system. Few other countries in the world use juries as we do in the United States.__6__the centuries, many people have believed that juries in most cases reach a fairer and more just result__7__would be obtained using a judge__8__, as many countries do.__9__a jury decides cases after “__10__”, or discussions among a group of people, the jury’s decision is likely to have the__11__ from many different people from different backgrounds, who must as a group decide what is right.
Juries are used in both civil cases, which decide__12__ among__13__ citizens, and criminal cases, which decide cases brought by the government __14__ that individuals have committed crimes. Juries are selected from the U.S. citizens and__15__. Jurors, consisting of __16__ numbers, are called for each case requiring a jury.
The judge__17__to the case__18__the selection of jurors to serve as the jury for that case. In some states,__19__jurors are questioned by the judge; in others, they are questioned by the lawyers representing the__20__under rules dictated by state law.
1. [A] other than [B] rather than [C] more than [D] or rather
2、化学性质:
I:稀硫酸(微粒: ),具有酸的通性。
II:浓硫酸(微粒:大部分是 分子),具有特殊性质
⑴吸水性:
常见的干燥剂:酸性的有_________;中性的有:_____;碱性的有______
 [图]常见的干燥装置:
[图]常见的干燥装置:



⑵脱水性:
[思考]①浓硫酸不小心沾到皮肤上如何处理?浓硫酸怎样稀释?
②浓硫酸滴在纸、棉花和木条上有何现象?
⑶强氧化性:(冷的浓硫酸遇Fe和Al要 )
① 氧化绝大部分金属(与CU、Fe等)
[实验]书P92:铜与浓硫酸反应装置
②
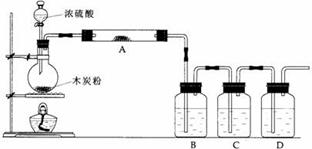 氧化非金属单质(C)
氧化非金属单质(C)
[例5]
(1)上述装置中,在反应前用手掌紧贴烧瓶外壁检查装置的气密性,如观察不到明显的现象,还可以用什么简单的方法证明该装置不漏气。
(2)如果用图中的装置检验上述反应的全部产物,写出下面标号所表示的仪器中应加入的试剂的名称及其作用:
A中加入的试剂是 ,作用是 。
B中加入的试剂是 ,作用是 。
C中加入的试剂是 ,作用是除尽 气体。
D中加入的试剂是 ,作用是 。
(4)实验时,C中应观察到的现象是 。
[思考]①蔗糖晶体中加入浓硫酸至过量产生的现象?这过程中浓硫酸体现了哪些性质?
②浓硫酸的氧化性与稀硫酸的氧化性的比较?浓、稀硫酸如何鉴别?
1、物理性质:无色,粘稠,密度大,高沸点, 挥发。
3.SO2的来源和酸雨(PH> )形成、危害 及其防治方法
[例3]关于酸雨的下列分析正确的是( )
A.因空气中无催化剂,SO2不能转化成SO3,酸雨中只含H2SO3
B.SO2在空气中有条件形成SO3,所以酸雨中含H2SO4
C.酸雨是无法避免的 D.硫在足量空气中燃烧成SO3,然后被水吸收形成酸雨
[例4]为防治酸雨,降低煤燃烧时向大气排放的SO2,工业上将生石灰和含硫煤混合使用。请写出燃烧时,有关“固硫”(不使含硫化合物进入大气)反应的化学方程式: 。
2.化学性质
(1)酸性氧化物(与CO2比较):酸性 H2SO3 H2CO3 HCO3-
①SO2与水反应:
②SO2与少量和足量的NaOH溶液:
③足量的SO2通入澄清石灰水中:
④足量的SO2通入碳酸钠溶液中: 。
(2)具有强还原性(与O2、X2的水溶液、KMnO4、HNO3等)
(3)具有弱氧化性(与H2S)
(4)具有漂白性:可使品红溶液褪色
[思考]SO2的漂白性与次氯酸的有何区别?
[例1]能证明SO2有漂白性的是 ( ) A. 酸性KMnO4溶液中通入SO2气体后紫色消失 B. 显红色的酚酞溶液通入SO2气体后红色消失 C. 品红溶液通入SO2气体后红色消失 D. 溴水通入SO2气体后橙色消失 [例2]SO2通入BaCl2溶液中,有白色沉淀?再加入FeCl3有何现象?该过程的离子方程式是___________________________。
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com